Review Article | Open Access
Exploring the effectiveness and safety profile of TAU protein antibodies as potential therapies for alzheimer's disease: a comprehensive review
Essa Muhammad1, Noor Ahmed1, Amanullah Kakar1, Wazir Akber1, Saleem Barech1, Anjum Farooq1, Tamour Mumtaz1
1Department of Neurology, Bolan Medical Complex Hospital, Quetta, Pakistan.
Correspondence: Syed Muhammad Essa (Department of Neurology, Bolan Medical Complex Hospital, Quetta, Pakistan; E-mail: royalbolan@gmail.com).
Asia-Pacific Journal of Surgical & Experimental Pathology 2024, 1: 1-12. https://doi.org/10.32948/ajsep.2024.03.28
Received: 18 Mar 2024 | Accepted: 26 Mar 2024 | Published online: 09 Apr 2024
Methodology This review investigates the safety and efficacy of TAU protein antibodies as possible treatments for AD. Using a variety of databases, a thorough literature search was carried out with an emphasis on clinical trials and academic publications regarding TAU protein antibodies in AD. Predetermined criteria were used to select eligible studies, and pertinent data were then retrieved and compiled. PRISMA guidelines for transparency were followed in the reporting.
Conclusion TAU protein antibodies have shown some potential in trials for treating Alzheimer's disease, including a little improvement in cognitive deterioration. Safety considerations highlight the need for cautious interpretation, especially with regard to imaging abnormalities due to amyloid. Optimizing efficacy, safety, and cost-effectiveness requires further studies.
Key words TAU protein, antibodies, alzheimer's disease, therapies
An essential pathogenic feature of Alzheimer's disease (AD) is the aberrant build-up of TAU protein aggregates in the brain, which is highly correlated with the degree of cognitive impairment [5]. In healthy neurons, TAU protein, a microtubule-associated protein, is essential for maintaining microtubule stability and promoting axonal transport [6, 7]. But TAU experiences abnormal post-translational changes in AD, which cause hyperphosphorylation and the consequent aggregation of tau into neurofibrillary tangles (NFTs) [8]. In Alzheimer's disease (AD), these NFTs impair neuronal function and exacerbate synaptic dysfunction, neuronal death, and cognitive decline [9].
Since TAU pathology has become a key component in the pathophysiology of AD, there is a great deal of interest in comprehending its function and investigating treatment approaches that target TAU aggregation and toxicity [10]. Although tau pathology has been difficult to detect in vivo, advances in biomarker research, such as blood-based biomarkers, positron emission tomography (PET), and cerebrospinal fluid assays, offer promising avenues for early diagnosis, disease monitoring, and treatment response assessment in AD [11, 12].
Numerous treatment strategies have been put forth to address the TAU pathology in AD, from small molecule inhibitors that target TAU phosphorylation and aggregation pathways to immunotherapies that try to remove TAU clumps [13, 14]. Despite these encouraging advancements, there are still many obstacles to overcome, such as the requirement for efficient blood-brain barrier penetration, specificity for pathogenic TAU species, and safety issues related to focusing on a protein that is so important to neuronal function.
TAU protein's typical role in the brain and its connection to microtubule stability:
The protein TAU is predominantly present in neurons and involved in the stabilization of microtubules, which are necessary for the preservation of cell shape, intracellular transport, and synaptic activity in neurons [7, 15]. When TAU is in its natural state, it attaches itself to microtubules, stabilizing them and preventing disintegration. The preservation of neurons' structural integrity and the maintenance of appropriate neural transmission depend on this function [16].
When TAU protein is altered abnormally in Alzheimer's disease (AD), it becomes hyperphosphorylated and separates from microtubules, aggregating into insoluble straight filaments (SFs) and paired helical filaments (PHFs and PHFs) [17]. Within neurons, these aggregated tau proteins build up to create what are called neurofibrillary tangles (NFTs) [18]. Axonal transport is hampered by the buildup of NFTs, which also causes disruption of regular cellular processes, neuronal malfunction, and ultimately cell death [19].
TAU pathology plays a significant role in the development and clinical presentation of AD [15]. There is a clear correlation between TAU pathology, especially the build-up of NFTs, and the development and clinical symptoms of AD [20]. According to studies, the development of tau pathology in the brain appears to follow a hierarchical pattern, beginning in particular brain regions related to memory formation and moving on to other brain regions as the disease advances [14]. Compared to amyloid-beta pathology, the degree and distribution of tau pathology in AD patients more closely correlates with cognitive impairment and the severity of the illness [21]. Furthermore, TAU pathology plays a crucial part in the pathophysiology of AD and Neurodegenerative diseases. These disorders are collectively referred to as tauopathies, and current study has demonstrated the importance of TAU pathology in these disorders.
Existing and emerging therapeutic strategies targeting TAU protein pathology in AD
A variety of strategies are used in therapeutic approaches targeting TAU pathology in Alzheimer's disease (AD) with the goal of addressing the underlying mechanisms of TAU protein dysfunction and aggregation [22, 23]. One strategy is the development of TAU aggregation inhibitors, which are designed to prevent the brain's formation of toxic TAU aggregates and neurofibrillary tangles (NFTs) by targeting specific sites on the TAU protein involved in the aggregation process and interfering with its pathological conformational changes [10, 24].
The use of monoclonal antibodies to target and eradicate pathogenic TAU species from the brain is known as tau immunotherapy, and it is another effective tactic [25]. These antibodies can either target particular phosphorylated epitopes linked to TAU pathology or directly bind to TAU clumps [26]. Immunotherapy seeks to reduce neurotoxicity and slow the development of AD disease by encouraging the removal of pathogenic TAU [27, 28].
Furthermore, vaccinations based on TAU protein are being investigated as a possible therapeutic strategy to encourage the production of antibodies by the immune system against TAU protein. Like traditional vaccination techniques, the goal of these vaccines is to elicit an immune response against pathogenic TAU aggregates [29].
Moreover, strategies for tau-targeted gene therapy are being researched to adjust the amount of TAU expressed or improve the brain's tau clearance pathways [30]. Potential methods for modifying TAU disease at the molecular level include gene therapy approaches like RNA interference targeting tau mRNA or the delivery of therapeutic genes via viral vectors [31, 32].
These therapeutic approaches have potential, but there are a number of issues and restrictions that need to be addressed. These include problems with the blood-brain barrier's penetration, the therapeutic agents' selectivity and specificity, the fluctuations in the disease's stage and course, the possibility of off-target effects, and safety issues including immunological reactions or neuroinflammation [32, 33].
The complex, multivariate character of AD pathology is also being addressed by multimodal approaches that combine TAU-targeted therapeutics with other therapy modalities, such as amyloid-targeted therapies or neuroprotective drugs [34, 35] (Figure 1).
Rationale of study
This study focuses on TAU protein pathology, a crucial component linked to the advancement of Alzheimer's disease (AD), in an effort to address the pressing need for efficient treatments for the condition. Despite its significance, there aren't many therapies that target TAU aggregation specifically. The purpose of this study is to thoroughly examine the safety and efficacy profile of TAU protein antibodies while taking into account their potential as therapeutic agents. The goal of this research is to enhance outcomes for AD patients by identifying knowledge gaps and integrating the available information to guide future treatment development efforts.
Objectives of the review
This comprehensive analysis aims to present an in-depth analysis of the efficacy and safety profile of TAU protein antibodies as prospective Alzheimer's disease therapeutics.
 Figure 1. Pathology of Alzheimer’s disease [61].
Figure 1. Pathology of Alzheimer’s disease [61].
In order to find pertinent research, we conducted an extensive literature search using electronic databases (PubMed, Embase, Google Scholar, and Cochrane Library). Relevant keywords including "TAU protein antibodies," "Alzheimer's disease," "therapeutics," and "clinical trials" were used in the search strategy. Only English-language articles published between the start of the search and the present were included. Figure 2 is the flowchart outlines for the reviews.
Inclusion and exclusion criteria
Articles were picked for inclusion according to predetermined standards. Included were original research publications, systematic reviews, meta-analyses, and clinical trials examining the safety or efficacy profile of tau protein antibodies in AD. Research presenting results pertaining to tau pathology, cognitive abilities, or unfavorable incidents were taken into account. Clinical trials using human and animal models could both be included.
Study selection process
The titles and abstracts of the retrieved papers were first used as a screening tool to find relevant studies. After that, the full-text publications of the chosen research were examined for eligibility. Two reviewers carried out the selection procedure separately, and discrepancies were settled through dialogue.
Data extraction and synthesis
From selected studies, we retrieved pertinent information on research design, participant characteristics, intervention specifics, outcome measures, and adverse events. We qualitatively summarized the data, highlighting patterns, discrepancies, and knowledge gaps.
Quality assessment
Depending on the study design, the appropriate instruments were used to evaluate the quality of the included studies. The Newcastle-Ottawa Scale was used for observational research, while the Cochrane Risk of Bias tool was used to assess the risk of bias in randomized controlled trials for clinical trials.
Ethical considerations
This review did not require ethical approval because it involves the analysis of data from previously published studies.
Reporting
To guarantee accuracy and transparency in the reporting of the review process, the approach followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
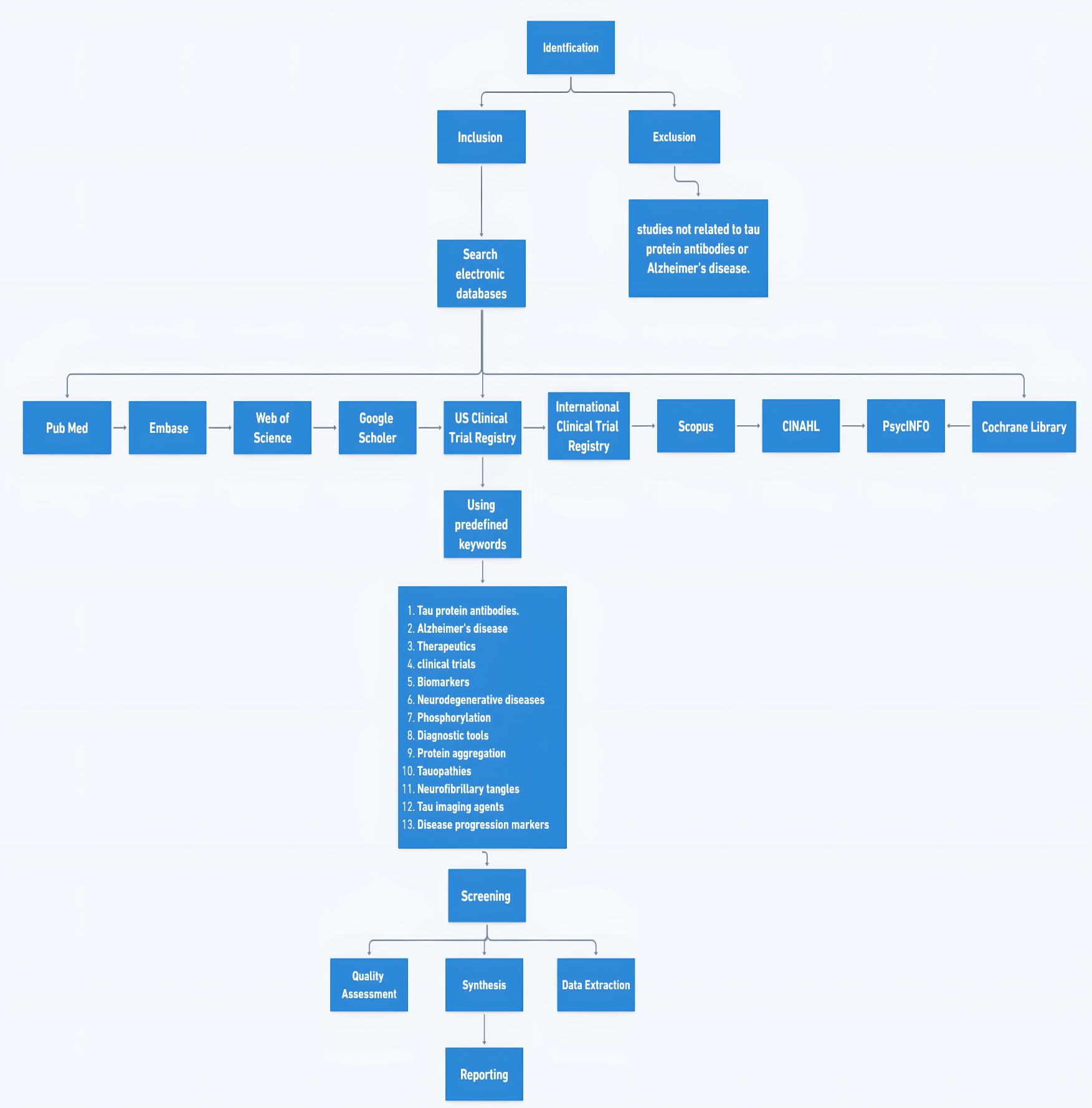 Figure 2. This flowchart outlines the steps involved in conducting a systematic literature review, including identifying relevant literature, conducting the literature search, screening titles and abstracts, assessing full-text articles for eligibility, including eligible studies in the review, data extraction, analysis, synthesis, and finally, preparing and writing the review paper.
Figure 2. This flowchart outlines the steps involved in conducting a systematic literature review, including identifying relevant literature, conducting the literature search, screening titles and abstracts, assessing full-text articles for eligibility, including eligible studies in the review, data extraction, analysis, synthesis, and finally, preparing and writing the review paper.
AADvac1 Immunotherapy (Petr Novak et al.): Preclinical and clinical research on AADvac1, an active immunotherapy that targets TAU pathology, has resulted in positive findings. Treatment with AADvac1 reduced neurofibrillary pathology and insoluble TAU in the brain and improved cognitive deficits in transgenic animal models expressing shortened, non-mutant TAU protein. Furthermore, it was discovered that AADvac1 stimulates the development of IgG antibodies against TAU peptides, making it a highly immunogenic drug in humans. In the brains of Alzheimer's patients, these antibodies were able to identify insoluble TAU proteins. Crucially, AADvac1 showed a good safety profile, with the main adverse event being injection site responses. AADvac1's potential as a disease-modifying treatment for tauopathies is currently being explored further through phase 1 trials for non-fluent primary progressive aphasia and phase 2 investigations for Alzheimer's disease [36] (Table 1).
Long-term Follow-up of AADvac1 Treatment (Petr Novak et al.): In patients with mild to severe Alzheimer's disease, a 72-week open-label study with AADvac1 showed both its long-term safety and immunogenicity. Booster doses were given to patients who had already had AADvac1 treatment, which raised their levels of IgG antibodies against tau peptides. With no incidences of meningoencephalitis or vasogenic edema reported, injection site reactions were the most frequent side effects seen [37]. Patients with higher IgG levels were notably shown to have a slower rate of cognitive decline and hippocampus atrophy, indicating that AADvac1 may have a protective impact against the advancement of the disease. These results encourage the ongoing assessment of AADvac1 in larger trials to completely determine its therapeutic efficacy.
ADAMANT Trial Results (Petr Novak et al.): AADvac1 was given in a phase 2 multicenter, placebo-controlled ADAMANT trial to patients with moderate AD dementia over 24 months. Eleven doses of AADvac1 were provided. AADvac1 showed a benign safety profile, with no discernible variations in adverse events between the groups receiving treatment and those receiving a placebo. High quantities of IgG antibodies were produced by the vaccination, but no discernible impacts on cognitive or functional outcomes were seen [38]. However, based on a slower rate of hippocampus atrophy and less cognitive deterioration, exploratory analysis revealed that individuals with greater IgG titers would benefit from AADvac1 (Table 2).
Liposome-based TAU vaccination (Clara Theunis et al.): Preclinical research on a liposome-based vaccination that targets phosphorylated TAU epitopes showed positive outcomes. In transgenic mice models of tauopathy, the vaccine elicited specific antisera and improved clinical conditions without causing neuroinflammation or unfavorable neurological consequences [39]. These results raise the possibility that liposome-based vaccinations could be used to safely and successfully treat tauopathies, which include Alzheimer's disease.
Novel Monoclonal Antibodies Targeting TAU Pathology (Jiaping Gu et al.): In brain slice cultures, two new monoclonal antibodies that specifically target the well-known 396/404 region of TAU dramatically reduced hyperphosphorylated soluble TAU without showing any signs of toxicity [40]. According to neurobiological studies, these antibodies were mainly absorbed by neurons and showed evidence of efficacy in removing TAU pathology by their colocalization with various pathogenic TAU markers [41]. These results validate the therapeutic significance of focusing on particular TAU epitopes in the management of tauopathies.
Anti-TAU Oligomer Immunization (Castillo et al.): The administration of an anti-TAU oligomer-specific antibody provided protection against the build-up of TAU oligomers and cognitive impairments in transgenic mice models of tauopathy (Castillo et al.) [39]. The promise of TAU oligomers as a therapeutic target for Alzheimer's disease and kindred tauopathies was highlighted by the antibody's ability to suppress oligomeric TAU while maintaining memory function after long-term administration.
Passive immunotherapy against TAU pathology
In the fight to create potent cures for Alzheimer's, passive immunotherapy against tau pathology appears to be an intriguing approach. Passive immunotherapy is the direct administration of premade antibodies that target particular tau epitopes, as opposed to active immunotherapy, which induces the body's immune system to create antibodies against abnormal TAU proteins (Table 3).
Taking on TAU pathology: tangle mouse PHF1 antibody
In female tangling mice, the study examines the efficacy of passive immunization with the PHF1 antibody (Allal Boutajangout et al) [42]. The results show a considerable decrease in TAU pathology in the hippocampal region and enhanced cognitive function. In the traverse beam task, the treated mice performed much better (p < 0.03) than the control group, and there was a significant 58% decrease in TAU pathology, notably in the dentate gyrus of the hippocampus (p = 0.02). The levels of insoluble pathogenic TAU were shown to have decreased by 14-27% (p < 0.05) and 34-45% (p < 0.05) for all TAU antibodies, according to Western blotting analysis. Furthermore, p < 0.05 significant relationships were found between TAU pathology, antibody levels, and behavioral performance [42].
Stopping the spread of TAU pathology: PHF6 and PHF13 antibodies
According to this study, TAU pathology induction and dissemination in both cell cultures and mice models can be prevented by using the phospho-TAU antibodies PHF6 and PHF13. These antibodies have the potential to mitigate TAU-related pathology and related cognitive deficits because they treated the tested models by improving memory and reducing the spread of TAU pathology [43].
RG7345: an overlooked possibility for TAU immunotherapy
An update is given regarding the progress made on the creation of the monoclonal antibody RG7345, which targets the tau phosphoepitope pS422. Clinical trials assessing RG7345 were stopped for undisclosed reasons despite encouraging preclinical evidence [44]. Anti-TAU/pS422 antibodies may provide therapeutic benefits in Alzheimer's disease and kindred tauopathies, but this is still up for debate [45].
BIIB092: efficacy assessment and safety profile
BIIB092, an anti-TAU antibody, was evaluated for safety, pharmacokinetics, and pharmacodynamic effects in individuals with Alzheimer's disease (AD) and progressive supranuclear palsy (PSP) [46]. In clinical trials for AD and PSP, BIIB092 did not reveal a meaningful efficacy, despite reducing extracellular TAU levels in cerebrospinal fluid and displaying an acceptable safety profile [47].
Gosuranemab safety in early Alzheimer's disease
Gosuranemab was tested in the TANGO study for its safety and ability to reduce levels of unbound N-terminal TAU in cerebrospinal fluid in a dose-dependent manner in patients with early Alzheimer's disease [48]. However, in comparison to the placebo group, no significant effects were observed in cognitive or functional tests, indicating that the intervention was ineffective in reducing cognitive decline and functional impairment in patients with early-stage Alzheimer's disease [49].
Semorinemab in prodromal to mild Alzheimer’s disease
As compared to a placebo, there was no discernible slowing of the illness's course in a phase 2 trial of semorinemab for prodromal to moderate Alzheimer's disease [50]. Although semorinemab had a satisfactory safety profile, there was no proof that it would improve cognitive or functional tests.
Lauriet study: semorinemab-induced cognitive decline reduction
When compared to a placebo, semorinemab significantly slowed the rate of cognitive decline in people with mild-to-moderate Alzheimer's disease, according to the Lauriet research [51]. On the other hand, functional outcomes showed no discernible impacts, and semorinemab showed a good safety profile.
Zagotenemab: safety and pharmacokinetics in Alzheimer's participants
Participants in a Phase Ib trial with mild to severe Alzheimer's disease or cognitive impairment were assessed for zagotenemab's safety and pharmacokinetics. Due to its low dose levels and short treatment period, zagotenemab did not exhibit significant pharmacodynamic changes, but it did reveal a safe profile with linear doses [52].
Zagotenemab in early symptomatic Alzheimer's disease
When compared to a placebo, zagotenemab did not significantly slow the course of early-stage Alzheimer's disease symptoms. Findings from imaging and plasma biomarkers also did not demonstrate any indication that the condition had changed, and those receiving zagotenemab medication reported a higher frequency of adverse events [53].
The potential to eliminate toxic TAU species with JNJ-63733657
JNJ-63733657 is a monoclonal antibody that targets phosphorylated TAU. It was tested for safety, pharmacokinetics, and pharmacodynamic effects in a randomized, double-blind, placebo-controlled study. The outcomes indicate that it may be able to eliminate toxic TAU species, which calls for more clinical research [54].
Efficacy and safety considerations of aducanumab
Despite debate over safety and efficacy, aducanumab was approved by the FDA in 2021 for the treatment of Alzheimer's disease based on encouraging surrogate marker data. There is interest in investigating further anti-amyloid treatments that may lessen the amyloid burden in AD which is in its early stages. Their influence on cognitive deterioration is still not clinically substantial, though. Cost-effectiveness and anomalies in imaging associated with amyloid are concerns. Extended follow-ups, combination therapy exploration, and better responder identification are recommended to increase their therapeutic relevance [55].
In 20 countries and 348 sites, 3,285 patients participated in two randomized phase 3 trials, ENGAGE and EMERGE. For 76 weeks, participants received aducanumab or a placebo intravenously (IV) every four weeks. EMERGE demonstrated a noteworthy enhancement in the primary results, whereas ENGAGE failed to achieve its goals. Alzheimer's disease markers decreased in both trials in a time- and dose-dependent manner. According to safety evaluations, edema associated with amyloid-related imaging abnormalities occurs most frequently [56].
PNT001: promising safety and pharmacokinetic profile
A phase 1 trial revealed encouraging safety and pharmacokinetic profiles for PNT001, a monoclonal antibody that targets phosphorylated TAU linked to Alzheimer's disease. The study indicated no safety complications and demonstrated target engagement in cerebrospinal fluid. It involved giving single ascending intravenous doses ranging from 900 to 4,000 mg to healthy individuals. These results provide credence to PNT001's possible development into other clinical trials for treating Alzheimer's disease [57] (Table 4).
Tilavonemab in early Alzheimer's disease
Despite having a generally well-tolerated safety profile, a phase 2 study of tilavonemab in early Alzheimer's disease found no benefit in decreasing cognitive decline when compared to placebo [58].
E2814
Preclinical investigations have demonstrated the potential of E2814, an IgG1 antibody intended to detect the TAU MTBR. The capacity of E2814 to prevent TAU aggregation and eradicate pathogenic TAU species is demonstrated by in vitro tests. E2814 can lessen the amount of TAU deposited in the brain, according to in vivo research that uses a TAU seeding model. The binding of E2814 to abnormal TAU structures in AD brain tissue is confirmed by immunohistochemical investigation. Additionally, compared to brains from other tauopathies or healthy controls, AD brains have higher amounts of TAU fragments having E2814 epitopes [59]. These results support the therapeutic potential of E2814 for AD and associated diseases (Table 5).
Lu AF87908
Lu AF87908 is a humanized mouse IgG1 monoclonal antibody that targets phosphorylated TAU proteins. Preclinical research showed that it can bind hyperphosphorylated TAU aggregates and inhibit TAU seeding and aggregation in mice models. In order to assess the safety and pharmacokinetics of Lu AF87908 in both healthy volunteers and Alzheimer's disease patients, a Phase 1 single-dose study was started in September 2019. A total of 86 adults from various cohorts were included in this placebo-controlled trial, which tracked the safety and plasma antibody concentrations for three months after infusion. Initial findings from preclinical research and the current Phase 1 trial lend support to the idea of investigating Lu AF87908 further as a possible treatment for tauopathies [60] (Figure 3).
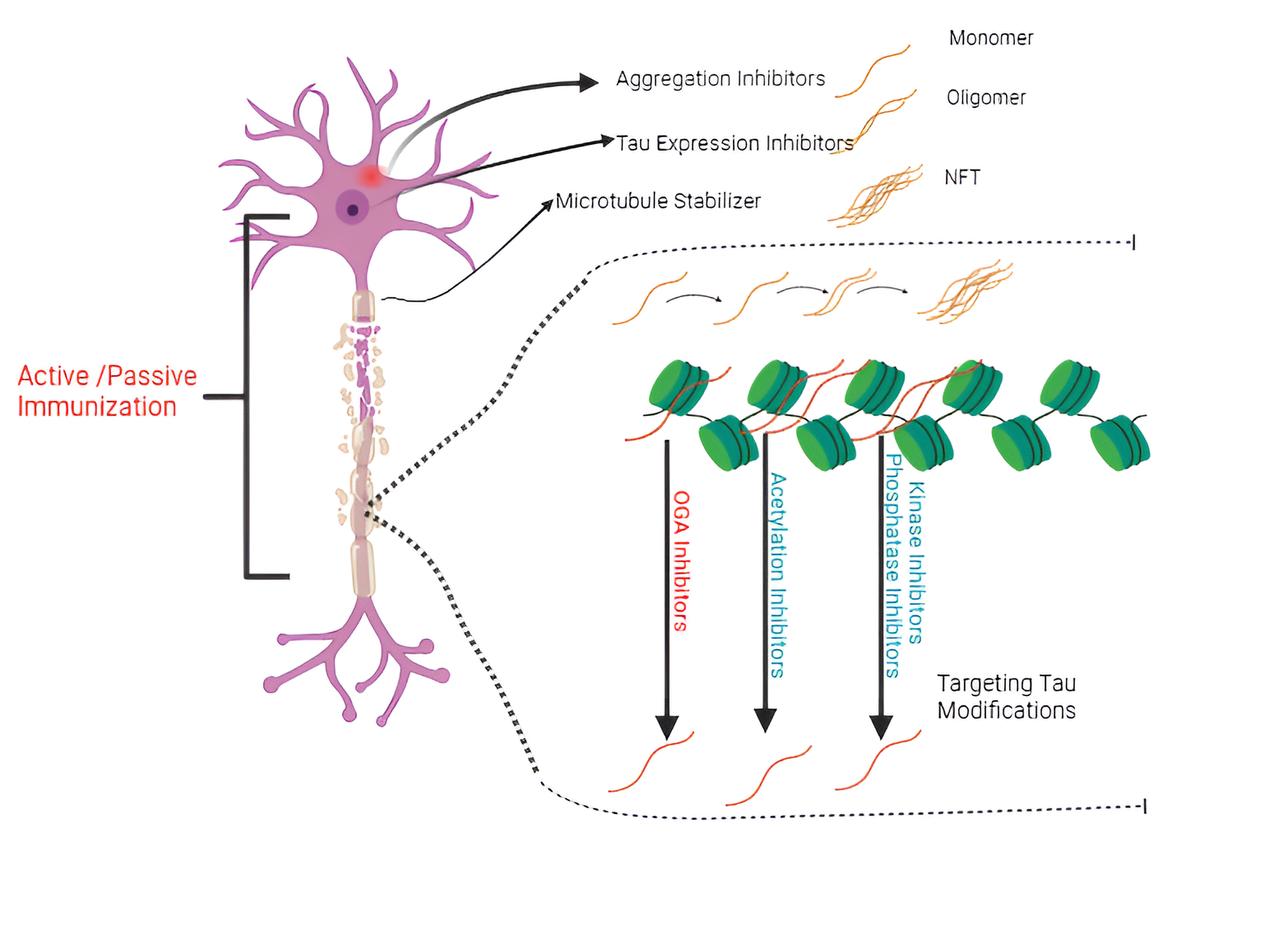 Figure 3. The approach to treating TAU protein involves several therapeutic strategies, primarily focusing on modifying TAU protein post-translational modifications (PTMs), preventing TAU protein aggregation and its expression, stabilizing microtubules, and employing immunotherapy techniques [60].
Figure 3. The approach to treating TAU protein involves several therapeutic strategies, primarily focusing on modifying TAU protein post-translational modifications (PTMs), preventing TAU protein aggregation and its expression, stabilizing microtubules, and employing immunotherapy techniques [60].
|
Table 1. Active immunotherapy. |
||||
|
Study |
Study design |
Intervention(s) |
Participants |
Outcome measures |
|
AADvac1 |
Preclinical study |
AADvac1 |
Transgenic rats and mice expressing TAU |
Reduction of neurofibrillary pathology and insoluble TAU, safety profile, immunogenicity in humans |
|
Petr Novak et al. |
Phase 1 study |
AADvac1 |
Patients with mild to moderate AD |
Safety, antibody titres, cognitive assessment, brain atrophy, lymphocyte populations |
|
Petr Novak et al. 2021 |
Phase 2 randomized trial |
AADvac1 |
Patients with mild AD dementia |
Safety, immunogenicity, efficacy in slowing cognitive and functional decline |
|
Clara Theunis et al. |
Preclinical study |
Liposome-based vaccine |
Wild-type mice and Tau.P301L mice |
Safety, efficacy in reducing tauopathy, clinical condition improvement |
|
Jiaping Gu et al. |
Preclinical study |
Monoclonal antibodies |
Transgenic mice brain slice cultures |
Safety, efficiency in clearing pathological TAU, mechanism of action in neurons |
|
Background and study aims |
Observational study |
ACI-35 |
Patients for vaccine safety testing |
Safety, antibody level evaluation |
|
Chai, X et al. |
Preclinical study |
Monoclonal antibodies |
Transgenic mouse models of TAU pathogenesis |
Reduction of biochemical TAU pathology, delay in motor function decline |
|
Castillo et al. |
Preclinical study |
Monoclonal antibody |
Wild-type and Htau mice |
Protection against TAU oligomer accumulation, cognitive deficits, memory function preservation |
|
Table 2. Adverse events reported in clinical studies. |
||||||
|
Study |
Total participants |
Injection site reactions |
Meningoencephalitis |
Vasogenic edema |
Micro-hemorrhages |
Other adverse events |
|
Petr Novak et al. |
26 |
13 (50%) |
0 |
0 |
1 case |
None reported |
|
Petr Novak et al. 2021 |
196 |
Not reported |
Not reported |
Not reported |
Not reported |
Not reported |
|
Table 3. Passive immunotherapy. |
||
|
Study title and authors |
Safety |
Efficacy |
|
Allal Boutajangout et al. [42] |
Improved performance on tasks; reduction in TAU pathology; decrease in insoluble TAU levels; significant correlations with behavior. |
Passive immunization with PHF1 antibody reduced TAU pathology and improved performance in tangle mice models. |
|
Sethu Sankaranarayanan et al. [43] |
Reduced spread of TAU pathology; improved memory tests. |
PHF6 and PHF13 antibodies mitigated TAU pathology and improved memory in neuron cultures and mouse models. |
|
Dr. Monica Neațu et al. [44] |
Favorable safety profile; one subject exhibited positive anti-drug antibody results. |
RG7345, targeting TAU phosphoepitope pS422, showed potential in preclinical studies but discontinued in clinical trials. |
|
Huang et al. [61] |
BIIB092 demonstrated acceptable safety; and dose-dependent reductions in tau levels. |
BIIB092 showed safety and dose-dependent reductions in tau levels but lacked efficacy in PSP and AD patients. |
|
Melanie Shulman et al. [48] |
Gosuranemab exhibited acceptable safety; and no significant cognitive effects. |
Gosuranemab showed safety and reduced TAU levels in cerebrospinal fluid but did not improve cognitive function in early AD. |
|
Edmond Teng et al.[50] |
Semorinemab showed acceptable safety; no significant treatment effects on cognition. |
Semorinemab did not slow disease progression in prodromal to mild AD but was well-tolerated. |
|
Cecilia Monteiro et al. [51] |
Semorinemab was well tolerated with favorable safety. |
Semorinemab showed a significant reduction in cognitive decline in mild-to-moderate AD with a favorable safety profile. |
|
Brian A. Willis et al. [52] |
Zagotenemab was safe but lacked significant pharmacodynamic effects. |
Zagotenemab was safe but did not slow clinical progression in early symptomatic AD and had a higher incidence of adverse events. |
|
Adam S. et al. [53] |
Zagotenemab treatment groups reported more adverse events than placebo. |
Zagotenemab did not slow clinical progression in early AD and exhibited a higher incidence of adverse events. |
|
Wendy R. Galpern et al. [56] |
JNJ-63733657 was safe and reduced TAU levels in cerebrospinal fluid. |
JNJ-63733657 demonstrated safety and dose-dependent reductions in tau levels, suggesting potential in clearing toxic TAU species. |
|
Alexander et al. [55] |
Not specified. |
Aducanumab approval led to controversy; other anti-amyloid immunotherapies are in trials, with safety and economic concerns. |
|
Wendy Luca et al. [57] |
PNT001 showed safety and promising pharmacokinetics in healthy volunteers. |
PNT001 showed safety and promising pharmacokinetics in healthy volunteers, supporting further clinical development. |
|
Erin E. Congdon et al. [62] |
IgG subclasses/subtypes exhibited varying efficacy and safety profiles; subtypes with effector function showed promise in reducing tau pathology. |
IgG subclasses/subtypes showed varying efficacy and safety profiles in TAU immunotherapy, with effector function showing promise in reducing tau pathology. |
|
Hana Florian et al. [58] |
Tilavonemab did not demonstrate efficacy in early AD despite acceptable safety and reductions in TAU levels. |
Tilavonemab did not demonstrate efficacy in early AD despite acceptable safety and reductions in TAU levels. |
|
Malcolm Roberts et al. [59] |
E2814 showed potential in inhibiting TAU aggregation and clearing pathological TAU, with favorable safety profiles. |
E2814, targeting the TAU MTBR, showed potential in preclinical studies for AD and other tauopathies by inhibiting TAU aggregation and clearing pathological TAU. |
|
Sigurdsson, Einar M. et al. [60] |
Preliminary data support further investigation of Lu AF87908 as a potential therapy for tauopathies, including AD. |
Lu AF87908 showed potential in preclinical studies for AD and other tauopathies. |
|
Table 4. Common findings across studies. |
|
|
Common findings |
Explanation |
|
Safety |
Not a single study found any significant safety issues during the trials; all indicated acceptable safety profiles for the studied antibodies in their respective patient populations. |
|
Lack of efficacy |
The majority of clinical trials did not show appreciable efficacy in terms of enhancing cognitive function or delaying the course of diseases, despite encouraging preclinical findings. |
|
Variable pharmacokinetics |
Antibodies exhibited distinct pharmacokinetic profiles, exhibiting variations in absorption, distribution, metabolism, and excretion among the substances under investigation. |
|
Target engagement |
Target engagement was validated by multiple studies, showing that the antibodies bonded to tau protein or amyloid-beta in preclinical and clinical contexts. |
|
Biomarker changes |
Amyloid-beta load and TAU levels in the cerebrospinal fluid (CSF) decreased after treatment with specific antibodies, two biomarkers linked to the pathophysiology of Alzheimer's disease. |
|
Lack of disease modification |
While some antibodies indicated decreases in biomarkers linked to the pathophysiology of Alzheimer's disease, none of the substances under investigation clearly demonstrated altered disease states in patients. |
|
Cognitive and functional measures |
Clinical trials frequently included cognitive and functional measures as primary or secondary endpoints, such as ADAS-Cog scores or Clinical Dementia Rating-Sum of Boxes (CDR-SB) scores. |
|
Discontinuation of development |
Despite showing early preclinical promise, some antibodies, including RG7345 and BIIB092, were pulled from further research due to less effectiveness or other reasons. |
|
Table 5. Preclinical studies overview. |
||||
|
Study title |
Animal model |
Treatment |
Duration |
Key findings |
|
Allal Boutajangout et al. |
Homozygous female tangle mice |
PHF1 antibody |
5-6 months |
Improved performance on traverse beam task, reduction in TAU pathology |
|
Sethu Sankaranarayanan et al. |
rTg4510 transgenic mouse |
PHF6 and PHF13 antibodies |
Not specified |
Reduced spread of TAU pathology, improvement in memory performance |
|
Dr. Monica Neațu et al. |
Human |
RG7345 |
1 year |
Discontinued; potential in reducing TAU pathology |
|
Melanie Shulman et al. |
Human |
Gosuranemab |
78 weeks |
Reduced TAU levels in CSF, no significant cognitive improvement |
|
Edmond Teng et al. |
Human |
Semorinemab |
73-week |
Well-tolerated, no significant treatment effects |
We are grateful to the Department of Neurology Bolan Medical University for the support rendered to ensure the success of this study.
Ethics approval
As the project was a review article no ethical approval was needed.
Data availability
All relevant data are within the paper.
Funding
The author(s) received no specific funding for this work.
Authors’ contribution
Essa Muhammad conceptualized and supervised the study; Noor Ahmed collected the data; Amanullah Kakar and Wazir Akber analysed the data; Saleem Barech and Anjum Farooq wrote the manuscript; Tamour Mumtaz proof read the manuscript.
Competing interests
The authors have declared that no competing interest exist.
- Self WK, Holtzman DM: Emerging diagnostics and therapeutics for Alzheimer disease. Nat Med 2023, 29(9): 2187-2199.
- Cacciamani F, Houot M, Gagliardi G, Dubois B, Sikkes S, Sánchez-Benavides G, Denicolò E, Molinuevo JL, Vannini P, Epelbaum S: Awareness of cognitive decline in patients with Alzheimer's disease: a systematic review and meta-analysis. Front Aging Neurosci 2021, 13: 697234.
- Rajan KB, Weuve J, Barnes LL, McAninch EA, Wilson RS, Evans DA: Population estimate of people with clinical Alzheimer's disease and mild cognitive impairment in the United States (2020-2060). Alzheimers Dement 2021, 17(12): 1966-1975.
- Imbimbo BP, Watling M: What have we learned from past failures of investigational drugs for Alzheimer’s disease? Expert Opin Investig Drugs 2021, 30(12): 1175-1182.
- Ratan Y, Rajput A, Maleysm S, Pareek A, Jain V, Pareek A, Kaur R, Singh G: An Insight into Cellular and Molecular Mechanisms Underlying the Pathogenesis of Neurodegeneration in Alzheimer’s Disease. Biomedicines 2023, 11(5): 1398.
- Cario A, Berger CL: Tau, microtubule dynamics, and axonal transport: New paradigms for neurodegenerative disease. Bioessays 2023, 45(8): 2200138.
- Jiménez JS: Macromolecular structures and proteins interacting with the microtubule associated tau protein. Neuroscience 2023, 518: 70-82.
- Bazrgar M, Khodabakhsh P, Mohagheghi F, Prudencio M, Ahmadiani A: Brain microRNAs dysregulation: Implication for missplicing and abnormal post-translational modifications of tau protein in Alzheimer’s disease and related tauopathies. Pharmacol Res 2020, 155: 104729.
- Zhang H, Jiang X, Ma L, Wei W, Li Z, Chang S, Wen J, Sun J, Li H: Role of Aβ in Alzheimer’s-related synaptic dysfunction. Front Cell Dev Biol 2022, 10: 964075.
- Giovannini J, Smeralda W, Jouanne M, Sopkova-de Oliveira Santos J, Catto M, Voisin-Chiret AS: Tau protein aggregation: key features to improve drug discovery screening. Drug Discov Today 2022, 27(5): 1284-1297.
- Ossenkoppele R, van der Kant R, Hansson O: Tau biomarkers in Alzheimer's disease: towards implementation in clinical practice and trials. Lancet Neurol 2022, 21(8): 726-734.
- Zhang L, Liang X, Zhang Z, Luo H: Cerebrospinal fluid and blood biomarkers in the diagnostic assays of Alzheimer’s disease. J Innov Optic Health Sci 2022, 15(01): 2230001.
- Peng Y, Jin H, Xue YH, Chen Q, Yao SY, Du MQ, Liu S: Current and future therapeutic strategies for Alzheimer’s disease: An overview of drug development bottlenecks. Front Aging Neurosci 2023, 15: 1206572.
- Limorenko G, Lashuel HA: Revisiting the grammar of Tau aggregation and pathology formation: how new insights from brain pathology are shaping how we study and target Tauopathies. Chem Soc Rev 2022, 51(2): 513-565.
- Muralidar S, Ambi SV, Sekaran S, Thirumalai D, Palaniappan B: Role of tau protein in Alzheimer's disease: The prime pathological player. Int J Biol Macromol 2020, 163: 1599-1617.
- Alquezar C, Arya S, Kao AW: Tau post-translational modifications: dynamic transformers of tau function, degradation, and aggregation. Front Neurol 2021, 11: 595532.
- Thwin AC: In-vitro propagation of pathological tau filaments related to Alzheimer’s Disease. San Francisco State University, 2022.
- Thal DR, Tomé SO: The central role of tau in Alzheimer’s disease: From neurofibrillary tangle maturation to the induction of cell death. Brain Res Bull 2022, 190: 204-217.
- Niewiadomska G, Niewiadomski W, Steczkowska M, Gasiorowska A: Tau oligomers neurotoxicity. Life 2021, 11(1): 28.
- Moore KBE, Hung TJ, Fortin JS: Hyperphosphorylated tau (p-tau) and drug discovery in the context of Alzheimer's disease and related tauopathies. Drug Discov today 2023, 28(3): 103487.
- Koychev I, Hofer M, Friedman N: Correlation of Alzheimer disease neuropathologic staging with amyloid and tau scintigraphic imaging biomarkers. J Nucl Med 2020, 61(10): 1413-1418.
- Ju Y, Tam KY: Pathological mechanisms and therapeutic strategies for Alzheimer’s disease. Neural Regen Res 2022, 17(3): 543-549.
- Congdon EE, Ji C, Tetlow AM, Jiang Y, Sigurdsson EM: Tau-targeting therapies for Alzheimer disease: Current status and future directions. Nat Rev Neurol 2023, 19(12): 715-736.
- Dominguez-Meijide A, Vasili E, Outeiro TF: Pharmacological modulators of tau aggregation and spreading. Brain Sci 2020, 10(11): 858.
- Congdon EE, Jiang Y, Sigurdsson EM: Targeting tau only extracellularly is likely to be less efficacious than targeting it both intra-and extracellularly. In: Seminars in Cell & Developmental Biology 2022, 126: 125-137.
- Gibbons GS, Kim SJ, Wu Q, Riddle DM, Leight SN, Changolkar L, Xu H, Meymand ES, O’Reilly M, Zhang B, et al: Conformation-selective tau monoclonal antibodies inhibit tau pathology in primary neurons and a mouse model of Alzheimer’s disease. Molecular Neurodegeneration 2020, 15(1): 64.
- Usman M, Bhardwaj S, Roychoudhury S, Kumar D, Alexiou A, Kumar P, Ambasta R, Prasher P, Shukla S, Upadhye V, et al: Immunotherapy for Alzheimer’s disease: current scenario and future perspectives. J Prev Alzheimers Dis 2021, 8(4): 534-551.
- Mortada I, Farah R, Nabha S, Ojcius DM, Fares Y, Almawi WY, Sadier NS: Immunotherapies for neurodegenerative diseases. Front Neurol 2021, 12: 654739.
- Plotkin SS, Cashman NR: Passive immunotherapies targeting Aβ and tau in Alzheimer's disease. Neurobiol Dis 2020, 144: 105010.
- Lennon MJ, Rigney G, Raymont V, Sachdev P: Genetic therapies for Alzheimer’s disease: a scoping review. J Alzheimers Dis 2021, 84(2): 491-504.
- Singh H, Das A, Khan MM, Pourmotabbed T: New insights into the therapeutic approaches for the treatment of tauopathies. Neural Regen Res 2024, 19(5): 1020-1026.
- Luo M, Lee LKC, Peng B, Choi CHJ, Tong WY, Voelcker NH: Delivering the promise of gene therapy with nanomedicines in treating central nervous system diseases. Adv Sci 2022, 9(26): 2201740.
- Passeri E, Elkhoury K, Morsink M, Broersen K, Linder M, Tamayol A, Malaplate C, Yen FT, Arab-Tehrany E: Alzheimer’s disease: Treatment strategies and their limitations. Int J Mol Sci 2022, 23(22): 13954.
- Ramesh M, Govindaraju T: Multipronged diagnostic and therapeutic strategies for Alzheimer's disease. Chem Sci 2022, 13(46): 13657-13689.
- Nguyen TT, Nguyen TTD, Nguyen TKO, Vo TK: Advances in developing therapeutic strategies for Alzheimer's disease. Biomedicine & Pharmacotherapy 2021, 139: 111623.
- Xia ZD, Ma RX, Wen JF, Zhai YF, Wang YQ, Wang FY, Liu D, Zhao XL, Sun B, Jia P, et al: Pathogenesis, Animal Models, and Drug Discovery of Alzheimer’s Disease. J Alzheimers Dis 2023, 94(4): 1265-1301.
- Alipour M, Tebianian M, Tofigh N, Taheri RS, Mousavi SA, Naseri A, Ahmadi A, Munawar N, Shahpasand K: Active immunotherapy against pathogenic Cis pT231-tau suppresses neurodegeneration in traumatic brain injury mouse models. Neuropeptides 2022, 96: 102285.
- Novak P, Kovacech B, Katina S, Schmidt R, Scheltens P, Kontsekova E, Ropele S, Fialova L, Kramberger M, Paulenka-Ivanovova N: ADAMANT: a placebo-controlled randomized phase 2 study of AADvac1, an active immunotherapy against pathological tau in Alzheimer’s disease. Nature Aging 2021, 1(6): 521-534.
- Castillo-Carranza DL, Gerson JE, Sengupta U, Guerrero-Muñoz MJ, Lasagna-Reeves CA, Kayed R: Specific targeting of tau oligomers in htau mice prevents cognitive impairment and tau toxicity following injection with brain-derived tau oligomeric seeds. J Alzheimers Dis 2014, 40(s1): S97-S111.
- Gu J, Congdon EE, Sigurdsson EM: Two novel Tau antibodies targeting the 396/404 region are primarily taken up by neurons and reduce Tau protein pathology. J Biol Chem 2013, 288(46): 33081-33095.
- Gu X, Qi L, Qi Q, Zhou J, Chen S, Wang L: Monoclonal antibody therapy for Alzheimer's disease focusing on intracerebral targets. Biosci Trends 2024, 18(1): 49-65.
- Boutajangout A, Ingadottir J, Davies P, Sigurdsson EM: Passive immunization targeting pathological phospho-tau protein in a mouse model reduces functional decline and clears tau aggregates from the brain. J Neurochem 2011, 118(4): 658-667.
- Sankaranarayanan S, Barten DM, Vana L, Devidze N, Yang L, Cadelina G, Hoque N, DeCarr L, Keenan S, Lin A, et al: Passive immunization with phospho-tau antibodies reduces tau pathology and functional deficits in two distinct mouse tauopathy models. PloS one 2015, 10(5): e0125614.
- Neațu M, Covaliu A, Ioniță I, Jugurt A, Davidescu EI, Popescu BO: Monoclonal Antibody Therapy in Alzheimer’s Disease. Pharmaceutics 2023, 16(1): 60.
- Collin L, Bohrmann B, Göpfert U, Oroszlan-Szovik K, Ozmen L, Grüninger F: Neuronal uptake of tau/pS422 antibody and reduced progression of tau pathology in a mouse model of Alzheimer‘s disease. Brain 2014, 137(10): 2834-2846.
- Ljubenkov PA, Vandevrede L, Rojas JC, Tsai R, Koestler M, Graham D, Dam T, Boxer AL: A phase 1b, randomized, double-blind, placebo-controlled, parallel cohort safety, tolerability, pharmacokinetics, pharmacodynamics and preliminary efficacy study of intravenously infused BIIB092 in patients with four different primary tauopathy syndromes. Alzheimer's & Dementia 2021, 17: e053116.
- Boxer AL, Qureshi I, Ahlijanian M, Grundman M, Golbe LI, Litvan I, Honig LS, Tuite P, McFarland NR, O'Suilleabhain P, et al: Safety of the tau-directed monoclonal antibody BIIB092 in progressive supranuclear palsy: a randomised, placebo-controlled, multiple ascending dose phase 1b trial. Lancet Neurol 2019, 18(6): 549-558.
- Shulman M, Kong J, O’Gorman J, Ratti E, Rajagovindan R, Viollet L, Huang E, Sharma S, Racine AM, Czerkowicz J, et al: TANGO: a placebo-controlled randomized phase 2 study of efficacy and safety of the anti-tau monoclonal antibody gosuranemab in early Alzheimer’s disease. Nat Aging 2023, 3(12): 1591-1601.
- Stern AM, Sperling RA: Tangles, not TANGO: targeting tau aggregates. Nat Aging 2023, 3(12): 1472-1473.
- Teng E, Manser PT, Pickthorn K, Brunstein F, Blendstrup M, Bohorquez SS, Wildsmith KR, Toth B, Dolton M, Ramakrishnan V, et al: Safety and efficacy of semorinemab in individuals with prodromal to mild Alzheimer disease: a randomized clinical trial. JAMA Neurol 2022, 79(8): 758-767.
- Monteiro C, Toth B, Brunstein F, Bobbala A, Datta S, Ceniceros R, Sanabria Bohorquez SM, Anania VG, Wildsmith KR, Schauer SP, et al: Randomized phase II study of the safety and efficacy of semorinemab in participants with mild-to-moderate Alzheimer disease: Lauriet. Neurology 2023, 101(14): e1391-e1401.
- Willis BA, Lo AC, Dage JL, Shcherbinin S, Chinchen L, Andersen SW, LaBell ES, Perahia DG, Hauck PM, Lowe SL: Safety, Tolerability, and Pharmacokinetics of Zagotenemab in Participants with Symptomatic Alzheimer’s Disease: A Phase I Clinical Trial. J Alzheimers Dis Rep 2023, 7(1): 1015-1024.
- Fleisher AS, Munsie LM, Perahia DG, Andersen SW, Higgins IA, Hauck PM, Lo AC, Sims JR, Brys M, Mintun M, et al: Assessment of Efficacy and Safety of Zagotenemab: Results From PERISCOPE-ALZ, a Phase 2 Study in Early Symptomatic Alzheimer Disease. Neurology 2024, 102(5): e208061.
- Galpern WR, Mercken M, Van Kolen K, Timmers M, Haeverans K, Janssens L, Triana-Baltzer G, Kolb HC, Jacobs T, Nandy P: P1-052: a single ascending dose study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of the anti-phospho-tau antibody JNJ-63733657 in healthy subjects. Alzheimer's & Dementia 2019, 15: P252-P253.
- Alexander GC, Emerson S, Kesselheim AS: Evaluation of aducanumab for Alzheimer disease: scientific evidence and regulatory review involving efficacy, safety, and futility. JAMA 2021, 325(17): 1717-1718.
- Budd Haeberlein S, Aisen P, Barkhof F, Chalkias S, Chen T, Cohen S, Dent G, Hansson O, Harrison K, Von Hehn C, et al: Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J Prev Alzheimers Dis 2022, 9(2): 197-210.
- Luca W, Foster K, McClure K, Ahlijanian M, Jefson M: A Phase 1 Single-Ascending-Dose Trial in Healthy Volunteers to Evaluate the Safety, Tolerability, Pharmacokinetics, and Immunogenicity of Intravenous PNT001, a Novel Mid-domain Tau Antibody Targeting cis-pT231 Tau. J Prev Alzheimers Dis 2024, 11(2): 366-374.
- Florian H, Wang D, Arnold SE, Boada M, Guo Q, Jin Z, Zheng H, Fisseha N, Kalluri HV, Rendenbach-Mueller B, et al: Tilavonemab in early Alzheimer’s disease: results from a phase 2, randomized, double-blind study. Brain 2023, 146(6): 2275-2284.
- Roberts M, Sevastou I, Imaizumi Y, Mistry K, Talma S, Dey M, Gartlon J, Ochiai H, Zhou Z, Akasofu S, et al: Pre-clinical characterisation of E2814, a high-affinity antibody targeting the microtubule-binding repeat domain of tau for passive immunotherapy in Alzheimer’s disease. Acta Neuropathol Commun 2020, 8(1): 13.
- Sigurdsson EM: Tau Immunotherapies for Alzheimer’s Disease and Related Tauopathies: Status of Trials and Insights from Preclinical Studies. J Alzheimers Dis 2024, 101(s1): S129-S140.
- Huang LK, Kuan YC, Lin HW, Hu CJ: Clinical trials of new drugs for Alzheimer disease: a 2020–2023 update. J Biomed Sci 2023, 30(1): 83.
- Congdon EE, Pan R, Jiang Y, Sandusky-Beltran LA, Dodge A, Lin Y, Liu M, Kuo MH, Kong XP, Sigurdsson EM: Single domain antibodies targeting pathological tau protein: Influence of four IgG subclasses on efficacy and toxicity. EBioMedicine 2022, 84: 104249.
Asia-Pacific Journal of Surgical & Experimental Pathology
ISSN 2977-5817 (Online)
 Copyright © Asia Pac J Surg Exp & Pathol. This
work is licensed under a Creative Commons AttributionNonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0)
License.
Copyright © Asia Pac J Surg Exp & Pathol. This
work is licensed under a Creative Commons AttributionNonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0)
License.

 Submit Manuscript
Submit Manuscript