Review Article | Open Access
Research advances in gut microbes and autism spectrum disorders
Renardo Lico1
1Departamenti i Neurologjisë, Spitali Amerikan 3, Tiranë, Albania.
Correspondence: Renardo Lico (Departamenti i Neurologjisë, Spitali Amerikan 3, Tiranë, Albania; E-mail: lico.renardo@icloud.com).
Asia-Pacific Journal of Surgical & Experimental Pathology 2024, 1: 71-80. https://doi.org/10.32948/ajsep.2024.11.25
Received: 08 Sep 2024 | Accepted: 05 Dec 2024 | Published online: 08 Dec 2024
Key words autism spectrum disorder, gut microbes, probiotics, bacterial community transplantation
With ongoing research, scientists have begun to recognise the significant role that gut microbiota plays in the health of the nervous system. This has led to increased interest in studying the relationship between the gut microbiota and ASD. The human gastrointestinal tract is home to a vast array of bacteria and other microorganisms, which not only assist in the digestion and metabolism of food, but also play vital roles in immune regulation, pathogen defense, and the overall health of the nervous system [4]. It has been shown that the gut and brain are interconnected through several pathways in the nervous, endocrine, and immune systems [5].
Numerous studies have demonstrated that the gut microbiome of individuals with ASD significantly differs from that of healthy individuals. For example, patients with ASD may experience an imbalance in their gut microbiota, with a higher relative abundance of bacteria from the phylum Firmicutes and a lower proportion from the phylum Bacteroidetes [6]. Furthermore, neurodevelopmental disorders in individuals with ASD may be linked to specific abnormalities in their gut flora, such as a reduction in Bifidobacterium and an increase in Clostridium species [7]. These microbial imbalances may affect brain function and host immune regulation through their influence on the synthesis of short-chain fatty acids, such as butyrate. In addition, alterations in certain metabolites in the gut have been found in patients with ASD, and these metabolites may influence behavior and brain development via the gut-brain axis pathway [8].
There is growing evidence that certain behavioral signs of autism are linked to gut microbes. Individuals with ASD are significantly more likely than the general population to experience common gastrointestinal symptoms such as diarrhea, constipation, and abdominal pain [9]. A significant correlation has been observed between the condition of the digestive tract and the deterioration of ASD symptoms, which is accompanied by dysbiosis of the gut microbiota [10]. Furthermore, researchers believe that the gut microbiota and their metabolites may influence the brain via the bloodstream or vagus nerve, thereby affecting patients' social behavior and cognitive function. This relationship has sparked new discussions about how gut health may influence brain cognition [11].
The gut-brain axis has become a key research topic in the study of the mechanisms underlying the relationship between the gut microbiota and autism. The complex network of metabolic functions, immune regulation, and neural transmission connects the gut and the brain. The central nervous system receives signals from the gut microbiota through the vagus nerve and enteric nervous system, which in turn influences emotions, behavior, and cognitive abilities [12]. Research has shown that Specific gut microbiota can affect the production of neurotransmitters through metabolic pathways, including gamma-aminobutyric acid (GABA) and 5-hydroxytryptamine (serotonin). These neurotransmitters are crucial for regulating emotions and behavior [13]. Behavioral problems and emotional disorders in individuals with ASD may be partially caused by changes in the abundance of microorganisms associated with neurotransmitter production and their functions in the gut [14].
In addition to the behavioral and metabolic correlations, gut microbiota also plays a major role in immune system regulation. One of the most concerning factors in the etiology of ASD is immune system dysfunction. Through the regulation of immune responses, gut microbiota may influence the inflammatory state of the central nervous system, which further exacerbates the behavioral symptoms of ASD [15]. For instance, certain pathogenic bacteria can increase intestinal permeability, leading to a "leaky gut," which allows inflammatory factors to enter the body and trigger disease. Reports indicate that the level of intestinal permeability in ASD patients is significantly higher than that in the general population, further supporting the potential link between the gut microbiota and ASD pathology [16].
Given these findings, gut microbiota therapy has attracted increasing interest as a potential treatment for ASD. Currently, therapies such as fecal microbiota transplantation, probiotics, and prebiotics have been shown to help alleviate behavioral symptoms in ASD patients. For example, Kang et al.'s experimental results indicated that fecal microbiota transplantation significantly reduced gastrointestinal symptoms in ASD patients and positively impacted social behavior [17]. Additionally, dietary interventions such as casein- and gluten-free diets have been shown to reduce behavioral symptoms in some ASD patients by altering the composition of the gut microbiota [18]. Despite these successes, individual variability remains significant, and there is limited research on the long-term safety and effectiveness of these therapies.
This review summarises recent research on the relationship between the gut microbiota and ASD, particularly its role in the pathophysiology of ASD and its potential as a therapeutic target. Through this review, we aim to provide a more comprehensive and in-depth perspective on the role of gut microbes in ASD and to offer a scientific basis for the exploration of future therapeutic options.
Recent studies have shown that the gut microbiota composition in individuals with ASD is markedly different from that in healthy individuals. This microbial imbalance may influence behavioral symptoms and neurological functioning. Generally, the gut microbiota in individuals with ASD is less diverse, which can lead to altered metabolite production and compromised gut barrier function, both of which can negatively affect brain development [19].
Studies have shown that the ratio of certain specific bacterial species in the gut of individuals with ASD is significantly altered. In a study by Finegold SM, it was reported that children with autism had nine types of Clostridia not found in controls, whereas the controls harbored only three species of Clostridia that were absent in children with autism, resulting in a total of 25 different Clostridia genera [20]. For example, individuals with ASD often have higher levels of Clostridium spp., Desulfovibrio spp., and Bacteroides spp., but much lower levels of certain probiotics, such as Bifidobacterium and Lactobacillus. According to Macfabe’s study, these alterations may lead to an increase in potentially neurotoxic metabolites, such as propionic acid, which is thought to be linked to behavioral abnormalities and cognitive dysfunction [21].
A more comprehensive analysis of the gut microbiota revealed that patients with ASD have a notably high percentage of Clostridium spp. Through the production of harmful chemicals or intestinal inflammation, this microbiota may affect the gut-brain axis [7]. For example, Fasano A. suggests that Clostridium and its byproducts contribute to increased intestinal permeability, leading to the "leaky gut" phenomenon that allows inflammatory substances to enter the bloodstream. This can trigger an inflammatory response in the brain and damage to the blood-brain barrier [22]. It is believed that this neuroinflammatory condition may play a role in the symptoms of ASD.
Advances in animal models and population studies
Scientists have employed a variety of methods to explore the connection between gut microbes and ASD, such as microbial composition studies based on human patients and animal model experiments. Fecal samples from ASD patients are often analyzed and compared with controls in population-based studies to identify microbial differences and potential associations [19]. In order to identify differences in specific bacterial strains between groups, 16S rRNA gene sequencing can be used to conduct high-throughput analysis of the gut microbial composition in both patients and healthy controls. According to Strati F.'s research, the diversity of gut microbiota in children with ASD significantly decreases, especially the abundance of specific bacteria like Bifidobacterium brevis and Lactobacillus, which undergoes significant changes. Research has shown that the role of these bacteria in immune regulation and metabolic pathways might be one of the key factors contributing to the occurrence and development of ASD [23]. Additionally, Wang M.'s research results also indicated that the gut microbiota of ASD patients may affect the metabolism of neurotransmitters such as glutamate, providing further insight into the potential mechanisms by which gut bacteria influence the nervous system in ASD patients through metabolic regulation [24].
Animal models, particularly sterile mice and human microbiota transplantation models, have proven essential in revealing the causal effects of microbiota on behavior [25]. By transplanting gut microbes from patients with autism into a mouse model, Hsiao et al. found that these mice exhibited autism-like behaviors, including social deficits and repetitive stereotyped behaviors [8]. Such studies not only demonstrate an association between gut microbes and ASD behavior, but also suggest a potential causal role for microbes in the pathology of ASD.
Furthermore, a large body of twin research has provided additional support for the association between gut microbes and ASD. These studies have found that even in genetically identical twins, the composition of gut microbes can differentially affect the severity of ASD symptoms, suggesting that the environment (including the microbiome) plays an important role in the development of autism [26].
Mechanisms involved in the gut-brain axis
There are several ways in which gut microorganisms may be linked to the neurobehavioral symptoms of ASD, but the most important is the gut-brain axis. Through metabolites, neurotransmitters, and immunological signaling, gut microbes can alter brain function. The gut-brain axis is an elaborate web of the immune, endocrine, and neurological systems (Figure 1).
One of the key mechanisms by which gut microorganisms influence brain function is through the modulation of neurotransmitters. Numerous gut microorganisms have the capacity to influence the production and metabolism of neurotransmitters, either directly or indirectly. For example, some gut bacteria produce the neurotransmitters 5-hydroxytryptamine (serotonin) and γ-aminobutyric acid (GABA), which are essential for mood regulation and cognitive function[13]. The study by Strandwitz P. identifies a family of gut bacteria, including butyrate-producing species in the Bacteroidetes phylum, that are capable of generating GABA. According to the study, the gut-brain axis pathway may enable gut bacteria to produce GABA, which could impact the host’s mood and cognitive abilities [27]. Furthermore, Clarke G. investigated the regulation of the 5-hydroxytryptamine system by gut microbes using a germ-free mouse model and found that the absence of gut microbes affected 5-hydroxytryptamine levels in the hippocampus of the mice and led to behavioral changes [28]. This finding suggests that gut microbes play a significant role in the regulation of neurotransmitters, which in turn, affects cognitive and behavioral functions in patients with ASD. This also implies that alterations in 5-hydroxytryptamine levels in patients with ASD may be closely linked to changes in their gut microbiota.
Through controlling the immune system, gut microorganisms affect the development and functioning of the neurological system. By influencing T-cell homeostasis and cytokine production, the gut microbiota can modify the systemic immune response and serve as a significant regulator of the host immune system. Specifically, commensal bacteria, such as Bifidobacterium bifidum, through the production of polysaccharide A (PSA), have been found by Mazmanian SK. to regulate the host immune system by balancing T cell subsets and reducing the production of pro-inflammatory cytokines, thus preventing immune system over-activation [29]. Increases in inflammatory cytokines are frequently observed in patients with ASD, which may be linked to the immune activation caused by gut microorganisms. These inflammatory substances can pass through the blood-brain barrier into the central nervous system, where they may exacerbate neuroinflammation and influence behavioral symptoms. Later, Hsiao EY demonstrated the connection between immune activation triggered by gut microbes and ASD-related behaviors using a mouse model of ASD. Increased levels of inflammatory factors have been found in ASD mice, accompanied by sustained activation of the immune system. Some behaviors associated with ASD were reduced by altering the gut microbiome, suggesting that the immune system might play a role in the pathophysiology of ASD [30]. These findings lend further support to the idea that gut microorganisms influence the immune system, indicating that inflammation caused by immune activation may impact neurological function in individuals with ASD.
Furthermore, the gut-brain axis depends on the permeability of the intestinal barrier, and individuals with ASD frequently experience leaky gut syndrome or increased intestinal permeability. ASD patients have significantly higher intestinal permeability than healthy controls, and this abnormality may have a genetic predisposition, according to de Magistris L.'s measurements of intestinal permeability in both individuals with ASD and their relatives. The study suggests that elevated intestinal permeability could influence neurodevelopment by promoting systemic immune activation [31]. When the intestinal barrier is compromised, bacteria and metabolites can easily pass through the intestinal wall into the bloodstream, and this inflammatory process may contribute significantly to the worsening of neurological symptoms in ASD patients.
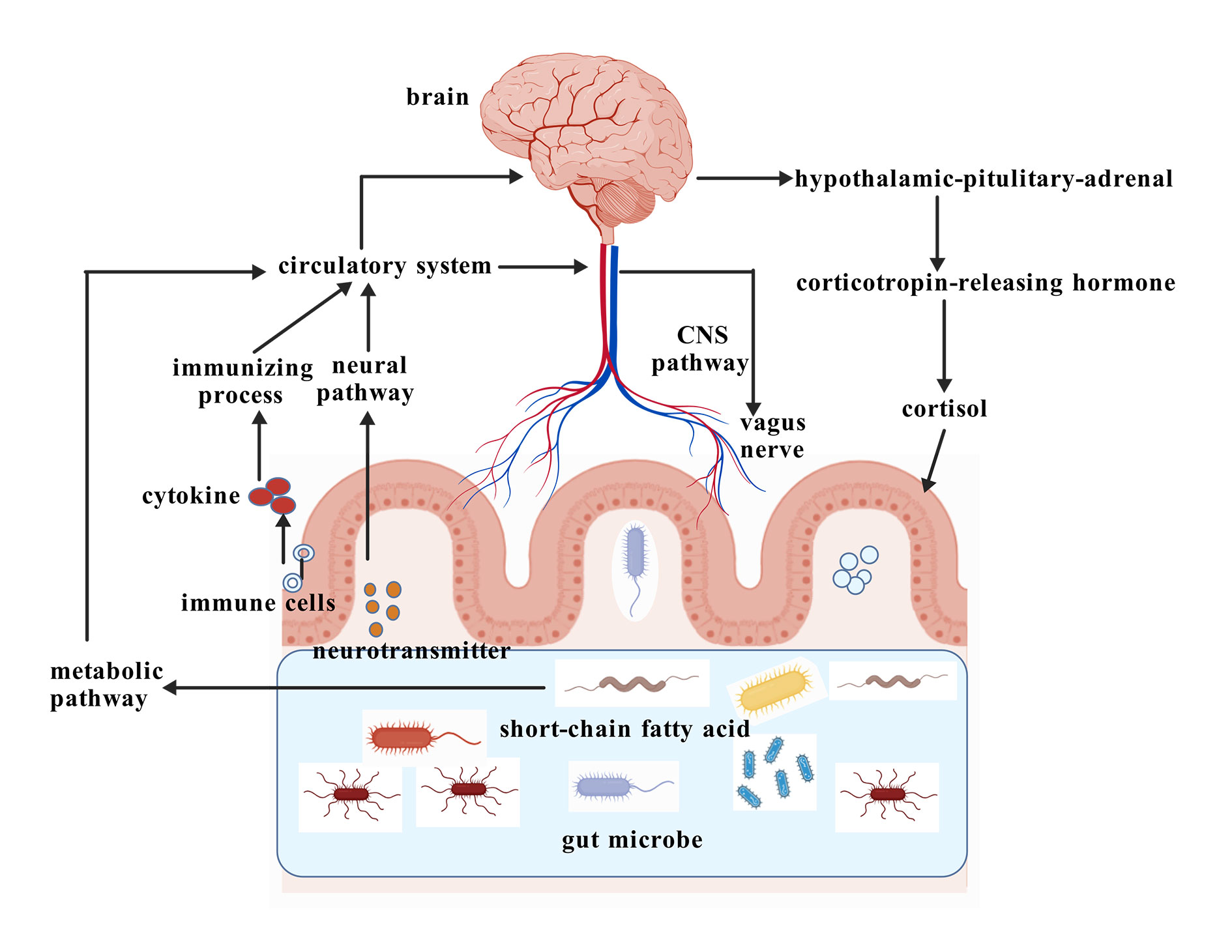 Figure 1. Regulation of gut brain axis.
Figure 1. Regulation of gut brain axis.
Relationship between the gut barrier and the brain barrier
The blood-brain barrier and the gut barrier work together to maintain the body’s internal environment, and the health of the blood-brain barrier is strongly influenced by the integrity of the gut barrier. Fiorentino M. discovered that individuals with ASD exhibit abnormal permeability in both the blood-brain barrier and the gut barrier, along with significantly higher serum levels of lipopolysaccharide (LPS) [32]. LPS is a potent immunostimulant that activates systemic immune responses and affects the intracerebral environment through the blood-brain barrier. This mechanism is thought to be closely linked to neuroinflammation and neurodevelopmental abnormalities in patients [32]. Ma B. later found that LPS levels were strongly associated with intestinal barrier damage by examining the gut microbiota and gut permeability in ASD patients [33]. Further research has shown that alterations in the gut microbiota and increased LPS levels can penetrate the blood-brain barrier, leading to intracerebral inflammation, which may impact the behavioral and cognitive functioning of individuals with autism.
Fasano et al. demonstrated that certain bacteria in the intestinal flora (e.g., Clostridium spp.) secrete a protein called zonulin, which regulates the permeability of both the intestinal and blood-brain barriers [34]. This allows inflammatory chemicals and bacterial products to pass from the gut to the brain, potentially resulting in neurological impairments. According to Esnafoglu E., patients with ASD had significantly higher serum levels of zonulin compared to control subjects, and this increase was associated with greater intestinal permeability. The study suggested that excessive zonulin levels may allow toxins and bacteria to pass through the intestinal barrier into the bloodstream, compromising the blood-brain barrier and potentially triggering an inflammatory response in the nervous system [35].
Neuroinflammation and immune regulation
The growing interest in the role of gut bacteria in neurodevelopment stems from the close connection between the immune and nervous systems. Immune system abnormalities are frequently observed in patients with ASD. For example, Ashwood et al. discovered that patients with ASD had significantly higher plasma levels of various pro-inflammatory cytokines such as IL-6 and TNF-α, indicating that the immune system is continuously activated. These immune system anomalies are correlated with behavioral deficiencies, which may affect neurodevelopment and behavioral performance in individuals with ASD[36]. Vargas DL. found that activation of microglia and astrocytes in the brains of ASD patients was accompanied by the expression of a large number of pro-inflammatory cytokines. These findings suggest that neuroinflammation in ASD patients may be caused by abnormal activation of the immune system, highlighting the crucial role of the immune system in the pathophysiological mechanisms of ASD [37].
Numerous studies have shown that some gut bacteria may influence immune system function by producing short-chain fatty acids (SCFAs), such as butyrate. When Frye RE et al. examined intestinal metabolites in patients with ASD found that levels of butyrate and other SCFAs were markedly lower. Reduced butyrate production may contribute to increased inflammation in both the gut and central nervous system, which could further impair neurodevelopment and behavioral abilities in patients with ASD [38]. Additionally, gut microbiota can affect immune system balance by regulating the activity of regulatory T cells (Tregs). In a mouse model of autism, Albekairi NA discovered that the CXCR2 antagonist SB332235 reduced behavioral impairments by upregulating the Treg-associated transcription factor signaling pathway and inhibiting Th1/Th22 cells [39]. Therefore, because CXCR2 antagonists have anti-inflammatory properties, they may represent a potential therapeutic approach to reduce behavioral impairments in ASD.
Influence of gut microorganisms during motherhood and infancy
The maternal-infant period is a critical stage of neurodevelopment, and the maternal gut microbiota has a significant impact on the development of both the immune system and the central nervous system of the fetus and infant. Changes in the maternal microbiota, particularly during pregnancy, have profound effects on the development of the immune system and the central nervous system in the offspring. For example, Vuong HE studied the effects of maternal gut microbiota during pregnancy on brain development in the offspring. According to this study, changes in the maternal gut microbiota during pregnancy affect neurotransmitters and metabolic pathways in the fetal brain, which in turn control neural development [40]. Additionally, Holingu's cohort study examining the relationship between antibiotic use during pregnancy or early infancy and the risk of neurodevelopmental disorders in children found that exposure to antibiotics during pregnancy was associated with an increased risk of neurodevelopmental problems, including ASD, in children. This study suggests that maternal antibiotic use may disrupt the maternal gut microbiota, leading to dysregulation in the development of the fetal brain and increased vulnerability to neurodevelopmental disorders such as ASD [41].
Moreover, pregnancy-related stress and nutritional changes can also alter the maternal gut microbiota, which can, in turn, influence the infant's gut flora through the placenta or breast milk. Zijlmans MA discovered that maternal psychological stress during pregnancy leads to alterations in the maternal gut microbiota, specifically a reduction in the abundance of health-related Bifidobacterium species. These changes in the maternal microbiota were transferred to the infant via the placenta or breast milk, potentially influencing the child’s immune system development [42]. Pannaraj's study demonstrated that the bacteria in breast milk are primarily staphylococci and streptococci, along with anaerobic Lactobacillus, Bifidobacterium, and Bacteroidetes species. These microbes present in breast milk serve as a significant source of microbiota for newborns [43]. The presence of similar microbial species in both breast milk and neonatal feces suggests that breast milk provides a key microbial inoculation to the infant’s gut [43, 44].
In addition, Henrik M. Roager et al. analyzed fecal samples from infants and children and found that breastfeeding promotes the production of aromatic lactic acids by Bifidobacterium bifidum in the infant’s intestines. These metabolites play an important role in shaping the immune function during early development [45]. By influencing immune function and regulating the gut-brain axis, these bacteria may affect the neurodevelopmental trajectory of children. Furthermore, Cho S et al. studied the interactions of human milk oligosaccharides (HMOs) with Bifidobacterium and Bacteroidetes species and their association with cognitive development in infants. Their findings showed that specific HMOs influence the composition of the infant gut microbiota and may in turn play a role in the cognitive development of infants [46]. Importantly, breast milk is rich in oligosaccharides, which act as prebiotics that promote the growth of beneficial gut microbiota. This, in turn, supports the development of the infant’s nervous system and may indirectly influence brain development and cognitive function [47].
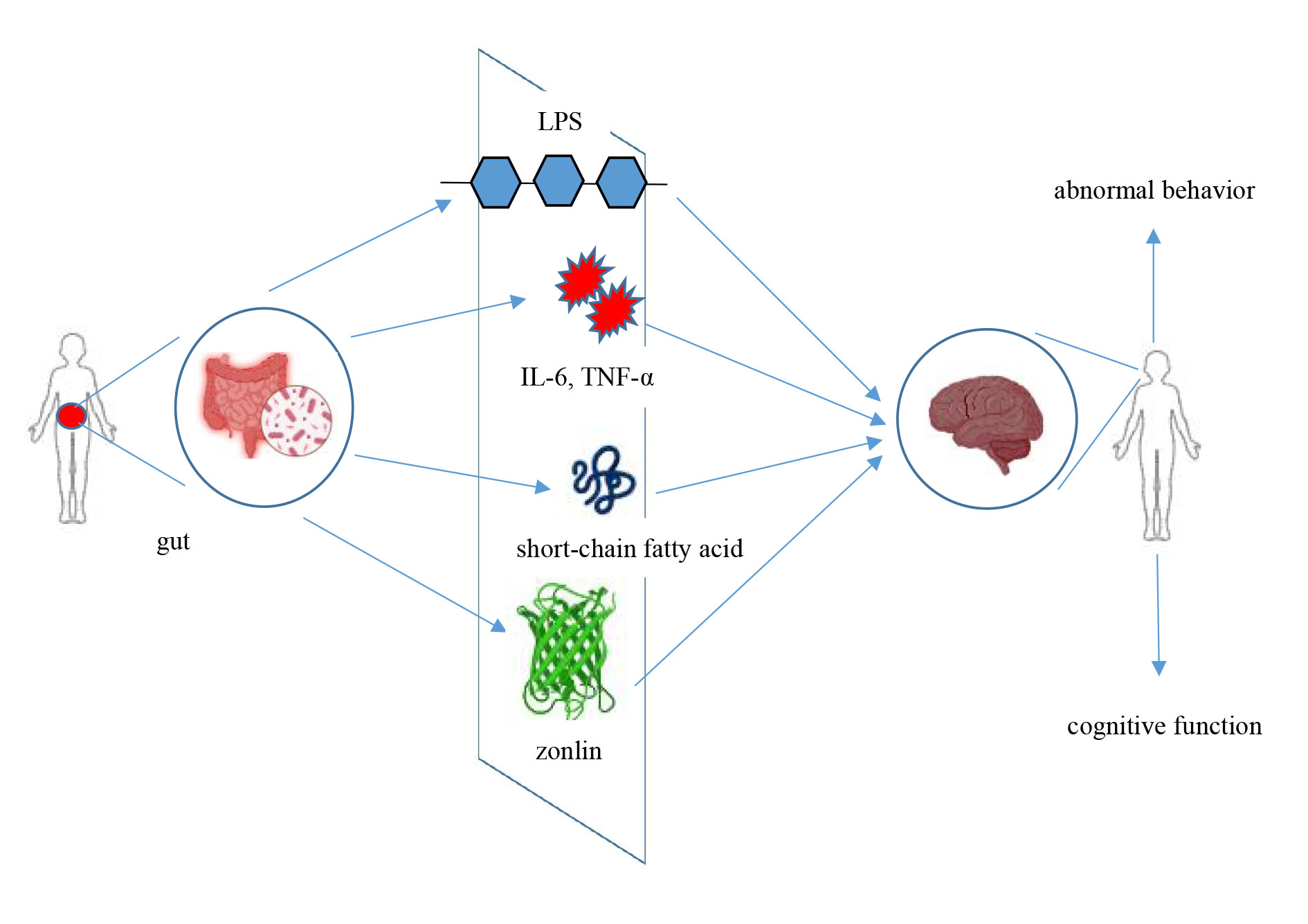 Figure 2. The mechanisms of gut microbes and autism.
Figure 2. The mechanisms of gut microbes and autism.
Application of probiotics and prebiotics
Recent studies suggest that certain probiotic strains may help alleviate symptoms in individuals with ASD. Probiotics are live microorganisms that provide health benefits to the host when consumed in adequate amounts. Mazzone L assessed the effects of two Lactobacillus reuteri strains (Lactobacillus reuteri ATCC-PTA-6475 and DSM-17938) when given as a combined product to children with ASD. The study found that while the probiotic treatment significantly improved the social behaviors of the children, it did not affect their immune profile, gut microbiome composition, restrictive/repetitive behaviors, or the overall severity of ASD symptoms [48].
In another study, Lin CH evaluated the effects of the probiotic bacterium Mimicronium fragilis BF839 in children with ASD. The results demonstrated that BF839 notably improved abnormal behaviors and gastrointestinal symptoms, especially motor skills related to stereotyped behaviors. The study also revealed that BF839 promoted the growth of beneficial Bifidobacterium species in the intestines of children with ASD [49].
Another probiotic, Bifidobacterium longum NCC3001, has been shown to alleviate anxiety and stress-related behaviors in a rat model by reducing the production of pro-inflammatory cytokines. Other probiotics may similarly influence inflammatory responses in individuals with ASD, potentially leading to a reduction in neurological symptoms through this regulatory effect on inflammation [50].
Prebiotics, which are indigestible food fibers, specifically promote the growth of beneficial gut microbes. Common prebiotics, such as galacto-oligosaccharides (GOS) and fructo-oligosaccharides (FOS), play a key role in regulating the composition of gut microbiota [51]. Studies have shown that prebiotics can help alleviate behavioral symptoms in individuals with ASD by increasing the abundance of beneficial flora (such as Bifidobacterium) and promoting the production of metabolites, including short-chain fatty acids, which support brain function [52].
Application of faecal bacterial transplantation
Fecal Microbial Transplantation (FMT) is a process where fecal flora from a healthy individual is transplanted into the gut of a patient, aiming to restore microbial diversity and reestablish a balanced gut flora. In patients with ASD, FMT has shown modest effectiveness. When the gut flora is enhanced using FMT, notable improvements in social behavior and emotional responses were observed in a mouse model of Autism Spectrum Disorder (ASD). Additionally, FMT reduced the levels of pro-inflammatory cytokines in the mice, indicating that it may play a role in controlling inflammation in the brain [53]. Several studies have applied FMT in various animal models with beneficial results [8, 17, 54-59] (Table 1).
Qin et al. explored the effects of FMT in children with ASD through a prospective study and found that after FMT, participants exhibited improvements in social interactions, emotional stability, and verbal communication. The study concluded that regulation of gut microbiota is a key factor in improving ASD symptoms [60]. Kang et al. conducted a study showing that FMT had therapeutic benefits for children with ASD. Their treatment regimen involved two weeks of intestinal cleansing and the use of oral antibiotics, including vancomycin, followed by FMT treatment for seven to eight weeks. The results showed that children who received FMT displayed significant changes in behavior and social skills, along with an increase in the diversity of their gut microbiota. Gastrointestinal issues, such as constipation, indigestion, and stomach discomfort, were also alleviated. These improvements in both behavior and gut health were sustained during a two-year follow-up after treatment [61]. The study further suggested that while FMT alters the gut ecology of patients, it may also modulate inflammation and neurotransmitter levels, thereby influencing behavior and cognitive abilities.
Although FMT shows potential as a therapeutic treatment for ASD, there are still many challenges in its clinical application. Further research is required to address its safety, strain selection, donor screening, and potential pathogenic risk. Therefore, more in-depth studies and careful clinical management are necessary to optimize the treatment protocol and ensure the safe use of FMT in patients with ASD.
Effect of dietary modifications on gut microbes and autism symptoms
Dietary modifications are a direct and effective method of influencing the gut microbiota, and in patients with ASD, certain dietary patterns have been shown to have a positive impact on symptoms. One of the most commonly used dietary patterns for ASD interventions is the Gluten-Free, Casein-Free (GFCF) diet. Many individuals with ASD exhibit sensitivities to gluten and casein, proteins found in wheat and dairy, respectively. These sensitivities may lead to increased intestinal permeability, which can trigger neuroinflammation and behavioral abnormalities. A study by Adams et al. found that some children with ASD showed improvements in social interactions, speech, and overall gut health after following a GFCF diet, suggesting that this dietary approach may be beneficial for symptom management [62].
Akhter M further supports the notion that a gluten-free, casein-free diet is safe and therapeutically effective for children with autism, indicating that a tailored dietary approach could be a viable management option. However, the existing trials related to the use of gluten-free and casein-free diets for children with autism are limited in number, often with small sample sizes and potential biases in results. Consequently, larger cohort studies with more rigorous designs are necessary to better understand the long-term therapeutic benefits of gluten-free and casein-free diets for children with ASD [63].
In addition to the GFCF diet, a high-fiber diet, which is rich in fruits, vegetables, and whole grains, has been shown to increase the number of beneficial bacteria in the gut, such as Bifidobacterium and Lactobacillus. These beneficial bacteria help improve the intestinal environment and contribute to the reduction of neuroinflammation by producing short-chain fatty acids (SCFAs) that have neuroprotective effects [64]. This type of diet may also contribute to overall gut health and play a role in alleviating some of the gastrointestinal and behavioral symptoms associated with ASD.
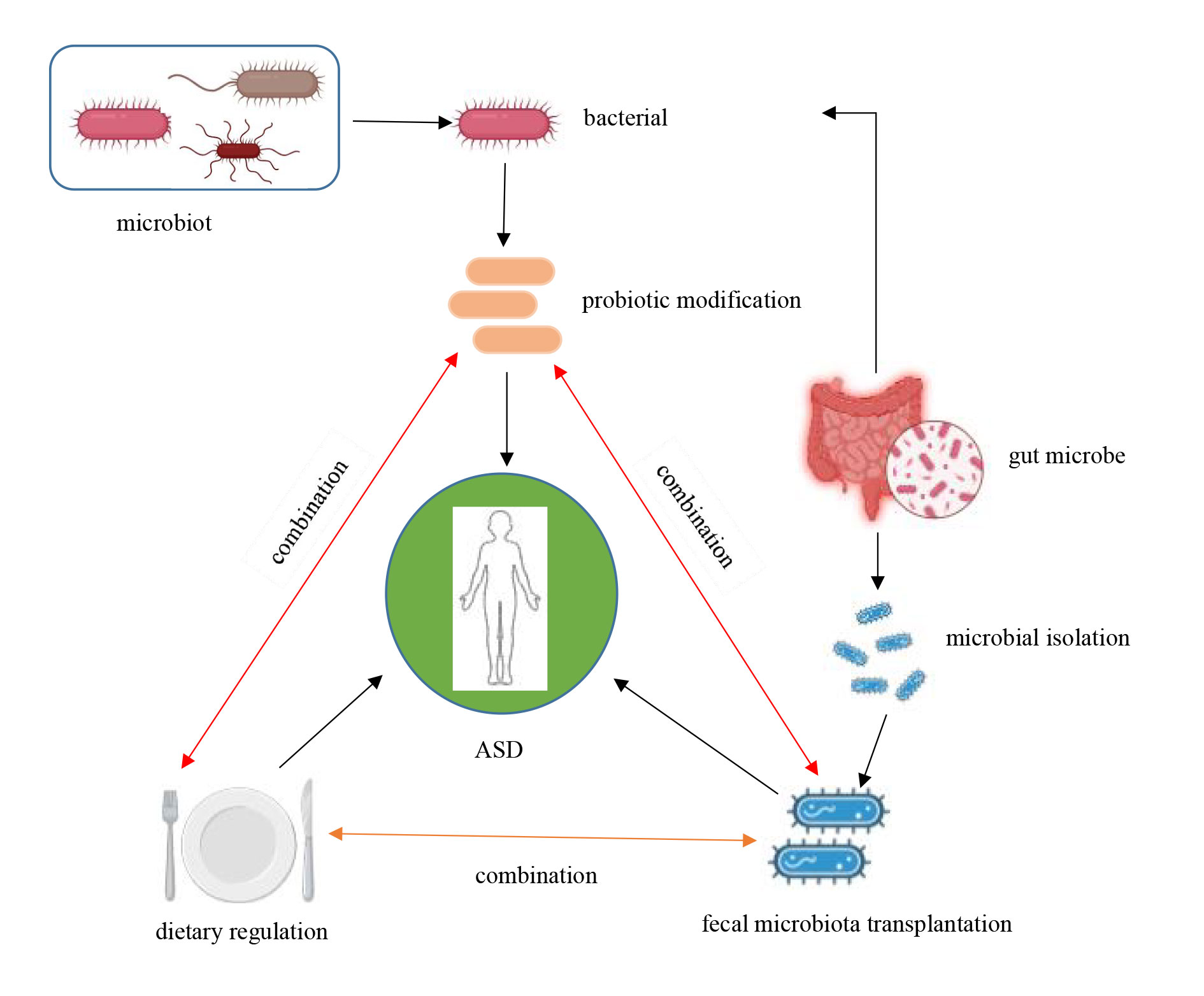 Figure 3. Treatment pathways for autism disorders.
Figure 3. Treatment pathways for autism disorders.
|
Table 1. Bacteriophage transplantation in animal models. |
|||
|
Animal Model |
FMT intervention methodology |
Key findings |
References |
|
Mice |
Obtaining fecal samples from healthy mice for transplantation |
FMT significantly improved social behavior and reduced stereotypic behavior in mice |
[18] |
|
Mice |
Transplantation of mice containing intestinal flora from ASD patients |
ASD-associated gut flora transplantation led to behavioral abnormalities and immune system changes and FMT restored some behavioral functions |
[54] |
|
BTBR mice (ASD model) |
Transplantation of feces from healthy mice to ASD model mice |
Improved social behaviors and reduced anxiety-like behaviors, moderated neuroinflammation |
[55, 56] |
|
Mice(ASD model) |
Healthy mouse samples for transplantation |
Restored some neurotransmitter levels, reduced repetitive behaviors, and increased short-chain fatty acid production |
[57, 58] |
|
Mouse (Lactobacillus reuteri intervention)) |
FMT combined with probiotic supplementation |
Improved social interaction and restored neural activity in relevant brain areas |
[59] |
|
Mice (induces ASD-like behavior) |
ASD model mice receive healthy mouse colony transplantation |
Improved behavioral abnormalities and reduced intestinal barrier permeability |
[8] |
The current study has several limitations, including sample size, standardization issues, and gaps in mechanistic understanding. The small sample size restricts the generalizability and reproducibility of the findings [63]. Additionally, the lack of a matched healthy control group diminishes the reliability of the results, while the heterogeneity of the gut microbiota and the absence of harmonized standards complicate the integration of findings from different studies. Furthermore, the exact mechanisms of the gut-brain axis in individuals with ASD remain unclear. Although extensive research has been conducted using animal models, translating these findings to humans presents challenges [65]. To address these limitations, future research should focus on large-scale, multicenter studies with standardized methodologies, integrated multi-omics approaches, and the exploration of personalized intervention strategies. Mechanistic studies that incorporate humanized models along with long-term follow-up could provide a more comprehensive foundation for treatment and offer potential pathways for intervention in ASD patients.
No applicable.
Ethics approval
No applicable.
Data availability
The data will be available upon request.
Funding
None.
Authors’ contribution
Renardo Lico contributed to the conception, design, writing of this review article and submitted the final version of the manuscript.
Competing interests
None.
- Wang L, Wang B, Wu C, Wang J, Sun M: Autism Spectrum Disorder: Neurodevelopmental Risk Factors, Biological Mechanism, and Precision Therapy. Int J Mol Sci 2023, 24(3): 1819.
- Lyall K, Schmidt RJ, Hertz-Picciotto I: Maternal lifestyle and environmental risk factors for autism spectrum disorders. Int J Epidemiol 2014, 43(2): 443-464.
- Sandin S, Lichtenstein P, Kuja-Halkola R, Hultman C, Larsson H, Reichenberg A: The Heritability of Autism Spectrum Disorder. Jama 2017, 318(12): 1182-1184.
- Structure, function and diversity of the healthy human microbiome. Nature 2012, 486(7402): 207-214.
- Mayer EA: Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci 2011, 12(8): 453-466.
- Kang DW, Park JG, Ilhan ZE, Wallstrom G, Labaer J, Adams JB, Krajmalnik-Brown R: Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One 2013, 8(7): e68322.
- Parracho HM, Bingham MO, Gibson GR, McCartney AL: Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J Med Microbiol 2005, 54(Pt 10): 987-991.
- Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, et al: Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155(7): 1451-1463.
- Buie T, Campbell DB, Fuchs GJ, 3rd, Furuta GT, Levy J, Vandewater J, Whitaker AH, Atkins D, Bauman ML, Beaudet AL, et al: Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics 2010, 125 Suppl 1: S1-18.
- Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA: Gastrointestinal flora and gastrointestinal status in children with autism--comparisons to typical children and correlation with autism severity. BMC Gastroenterol 2011, 11: 22.
- Collins SM, Bercik P: The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology 2009, 136(6): 2003-2014.
- Cryan JF, Dinan TG: Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 2012, 13(10): 701-712.
- Strandwitz P: Neurotransmitter modulation by the gut microbiota. Brain Res 2018, 1693(Pt B): 128-133.
- Sharon G, Sampson TR, Geschwind DH, Mazmanian SK: The Central Nervous System and the Gut Microbiome. Cell 2016, 167(4): 915-932.
- Mangiola F, Ianiro G, Franceschi F, Fagiuoli S, Gasbarrini G, Gasbarrini A: Gut microbiota in autism and mood disorders. World J Gastroenterol 2016, 22(1): 361-368.
- de Theije CG, Koelink PJ, Korte-Bouws GA, Lopes da Silva S, Korte SM, Olivier B, Garssen J, Kraneveld AD: Intestinal inflammation in a murine model of autism spectrum disorders. Brain Behav Immun 2014, 37: 240-247.
- Kang DW, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, Khoruts A, Geis E, Maldonado J, McDonough-Means S, et al: Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome 2017, 5(1): 10.
- Zafirovski K, Aleksoska MT, Thomas J, Hanna F: Impact of Gluten-Free and Casein-Free Diet on Behavioural Outcomes and Quality of Life of Autistic Children and Adolescents: A Scoping Review. Children (Basel) 2024, 11(7): 862.
- Coretti L, Paparo L, Riccio MP, Amato F, Cuomo M, Natale A, Borrelli L, Corrado G, Comegna M, Buommino E, et al: Gut Microbiota Features in Young Children With Autism Spectrum Disorders. Front Microbiol 2018, 9: 3146.
- Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen ML, Bolte E, McTeague M, Sandler R, Wexler H, Marlowe EM, et al: Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis 2002, 35(Suppl 1): S6-S16.
- Macfabe DF: Short-chain fatty acid fermentation products of the gut microbiome: implications in autism spectrum disorders. Microb Ecol Health Dis 2012, 23.
- Fasano A: Intestinal permeability and its regulation by zonulin: diagnostic and therapeutic implications. Clin Gastroenterol Hepatol 2012, 10(10): 1096-1100.
- Strati F, Cavalieri D, Albanese D, De Felice C, Donati C, Hayek J, Jousson O, Leoncini S, Renzi D, Calabrò A, et al: New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 2017, 5(1): 24.
- Wang M, Wan J, Rong H, He F, Wang H, Zhou J, Cai C, Wang Y, Xu R, Yin Z, et al: Alterations in Gut Glutamate Metabolism Associated with Changes in Gut Microbiota Composition in Children with Autism Spectrum Disorder. mSystems 2019, 4(1): e00321-18.
- Diaz Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S: Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A 2011, 108(7): 3047-3052.
- Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, Beaumont M, Van Treuren W, Knight R, Bell JT, et al: Human genetics shape the gut microbiome. Cell 2014, 159(4): 789-799.
- Strandwitz P, Kim KH, Terekhova D, Liu JK, Sharma A, Levering J, McDonald D, Dietrich D, Ramadhar TR, Lekbua A, et al: GABA-modulating bacteria of the human gut microbiota. Nat Microbiol 2019, 4(3): 396-403.
- Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF: The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry 2013, 18(6): 666-673.
- Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL: An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005, 122(1): 107-118.
- Hsiao EY, McBride SW, Chow J, Mazmanian SK, Patterson PH: Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc Natl Acad Sci U S A 2012, 109(31): 12776-12781.
- de Magistris L, Familiari V, Pascotto A, Sapone A, Frolli A, Iardino P, Carteni M, De Rosa M, Francavilla R, Riegler G, et al: Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J Pediatr Gastroenterol Nutr 2010, 51(4): 418-424.
- Fiorentino M, Sapone A, Senger S, Camhi SS, Kadzielski SM, Buie TM, Kelly DL, Cascella N, Fasano A: Blood-brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol Autism 2016, 7: 49.
- Ma B, Liang J, Dai M, Wang J, Luo J, Zhang Z, Jing J: Altered Gut Microbiota in Chinese Children With Autism Spectrum Disorders. Front Cell Infect Microbiol 2019, 9: 40.
- Fasano A: Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev 2011, 91(1): 151-175.
- Esnafoglu E, Cırrık S, Ayyıldız SN, Erdil A, Ertürk EY, Daglı A, Noyan T: Increased Serum Zonulin Levels as an Intestinal Permeability Marker in Autistic Subjects. J Pediatr 2017, 188: 240-244.
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J: Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun 2011, 25(1): 40-45.
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA: Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol 2005, 57(1): 67-81.
- Frye RE, DeLatorre R, Taylor HB, Slattery J, Melnyk S, Chowdhury N, James SJ: Metabolic effects of sapropterin treatment in autism spectrum disorder: a preliminary study. Transl Psychiatry 2013, 3(3): e237.
- Albekairi NA, Nadeem A, Ansari MA, Attia SM, Bakheet SA, Alanazi MM, Alhamed AS, Albekairi TH, Al-Mazroua HA, Ibrahim KE, et al: CXCR2 antagonist SB332235 mitigates deficits in social behavior and dysregulation of Th1/Th22 and T regulatory cell-related transcription factor signaling in male BTBR T(+) Itpr3(tf)/J mouse model of autism. Pharmacol Biochem Behav 2022, 217: 173408.
- Vuong HE, Pronovost GN, Williams DW, Coley EJL, Siegler EL, Qiu A, Kazantsev M, Wilson CJ, Rendon T, Hsiao EY: The maternal microbiome modulates fetal neurodevelopment in mice. Nature 2020, 586(7828): 281-286.
- Holingue C, Brucato M, Ladd-Acosta C, Hong X, Volk H, Mueller NT, Wang X, Fallin MD: Interaction between Maternal Immune Activation and Antibiotic Use during Pregnancy and Child Risk of Autism Spectrum Disorder. Autism Res 2020, 13(12): 2230-2241.
- Zijlmans MA, Korpela K, Riksen-Walraven JM, de Vos WM, de Weerth C: Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology 2015, 53: 233-245.
- Pannaraj PS, Li F, Cerini C, Bender JM, Yang S, Rollie A, Adisetiyo H, Zabih S, Lincez PJ, Bittinger K, et al: Association Between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatr 2017, 171(7): 647-654.
- Duranti S, Lugli GA, Mancabelli L, Armanini F, Turroni F, James K, Ferretti P, Gorfer V, Ferrario C, Milani C, et al: Maternal inheritance of bifidobacterial communities and bifidophages in infants through vertical transmission. Microbiome 2017, 5(1): 66.
- Laursen MF, Sakanaka M, von Burg N, Mörbe U, Andersen D, Moll JM, Pekmez CT, Rivollier A, Michaelsen KF, Mølgaard C, et al: Bifidobacterium species associated with breastfeeding produce aromatic lactic acids in the infant gut. Nat Microbiol 2021, 6(11): 1367-1382.
- Cho S, Samuel TM, Li T, Howell BR, Baluyot K, Hazlett HC, Elison JT, Zhu H, Hauser J, Sprenger N, et al: Interactions between Bifidobacterium and Bacteroides and human milk oligosaccharides and their associations with infant cognition. Front Nutr 2023, 10: 1216327.
- Zhong Z, Tang H, Shen T, Ma X, Zhao F, Kwok LY, Sun Z, Bilige M, Zhang H: Bifidobacterium animalis subsp. lactis Probio-M8 undergoes host adaptive evolution by glcU mutation and translocates to the infant's gut via oral-/entero-mammary routes through lactation. Microbiome 2022, 10(1): 197.
- Mazzone L, Dooling SW, Volpe E, Uljarević M, Waters JL, Sabatini A, Arturi L, Abate R, Riccioni A, Siracusano M, et al: Precision microbial intervention improves social behavior but not autism severity: A pilot double-blind randomized placebo-controlled trial. Cell Host Microbe 2024, 32(1): 106-116.e106.
- Lin CH, Zeng T, Lu CW, Li DY, Liu YY, Li BM, Chen SQ, Deng YH: Efficacy and safety of Bacteroides fragilis BF839 for pediatric autism spectrum disorder: a randomized clinical trial. Front Nutr 2024, 11: 1447059.
- Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, Blennerhassett PA, Fahnestock M, Moine D, et al: The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil 2011, 23(12): 1132-1139.
- Ladirat SE, Schoterman MH, Rahaoui H, Mars M, Schuren FH, Gruppen H, Nauta A, Schols HA: Exploring the effects of galacto-oligosaccharides on the gut microbiota of healthy adults receiving amoxicillin treatment. Br J Nutr 2014, 112(4): 536-546.
- Momo Cabrera P, Rachmühl C, Derrien M, Bourdet-Sicard R, Lacroix C, Geirnaert A: Comparative prebiotic potential of galacto- and fructo-oligosaccharides, native inulin, and acacia gum in Kenyan infant gut microbiota during iron supplementation. ISME Commun 2024, 4(1): ycae033.
- Li Q, Han Y, Dy ABC, Hagerman RJ: The Gut Microbiota and Autism Spectrum Disorders. Front Cell Neurosci 2017, 11: 120.
- Sharon G, Cruz NJ, Kang DW, Gandal MJ, Wang B, Kim YM, Zink EM, Casey CP, Taylor BC, Lane CJ, et al: Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell 2019, 177(6): 1600-1618.e17.
- Ye J, Fan H, Shi R, Song G, Wu X, Wang D, Xia B, Zhao Z, Zhao B, Liu X, et al: Dietary lipoic acid alleviates autism-like behavior induced by acrylamide in adolescent mice: the potential involvement of the gut-brain axis. Food Funct 2024, 15(7): 3395-3410.
- Gudka R, Nyinoh IW: Fecal microbial transplantation as a novel therapeutic for autism spectrum disorders: a review of the current literature. Front Microbiomes 2023, 2: 1222089.
- Li Y, Luo ZY, Hu YY, Bi YW, Yang JM, Zou WJ, Song YL, Li S, Shen T, Li SJ, et al: The gut microbiota regulates autism-like behavior by mediating vitamin B(6) homeostasis in EphB6-deficient mice. Microbiome 2020, 8(1): 120.
- Lai Y, Liu CW, Yang Y, Hsiao YC, Ru H, Lu K: High-coverage metabolomics uncovers microbiota-driven biochemical landscape of interorgan transport and gut-brain communication in mice. Nat Commun 2021, 12(1): 6000.
- Vuong HE, Hsiao EY: Emerging Roles for the Gut Microbiome in Autism Spectrum Disorder. Biol Psychiatry 2017, 81(5): 411-423.
- Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, et al: A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464(7285): 59-65.
- Kang DW, Adams JB, Coleman DM, Pollard EL, Maldonado J, McDonough-Means S, Caporaso JG, Krajmalnik-Brown R: Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Sci Rep 2019, 9(1): 5821.
- Adams JB, Audhya T, Geis E, Gehn E, Fimbres V, Pollard EL, Mitchell J, Ingram J, Hellmers R, Laake D, et al: Comprehensive Nutritional and Dietary Intervention for Autism Spectrum Disorder-A Randomized, Controlled 12-Month Trial. Nutrients 2018, 10(3): 369.
- Akhter M, Khan SM, Firdous SN, Tikmani P, Khan A, Rafique H: A narrative review on manifestations of gluten free casein free diet in autism and autism spectrum disorders. J Pak Med Assoc 2022, 72(10): 2054-2060.
- Pendyala S, Walker JM, Holt PR: A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology 2012, 142(5): 1100-1101.e2.
- Feng P, Zhao S, Zhang Y, Li E: A review of probiotics in the treatment of autism spectrum disorders: Perspectives from the gut-brain axis. Front Microbiol 2023, 14: 1123462.
Asia-Pacific Journal of Surgical & Experimental Pathology
ISSN 2977-5817 (Online)
 Copyright © Asia Pac J Surg Exp & Pathol. This
work is licensed under a Creative Commons AttributionNonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0)
License.
Copyright © Asia Pac J Surg Exp & Pathol. This
work is licensed under a Creative Commons AttributionNonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0)
License.

 Submit Manuscript
Submit Manuscript