Review Article | Open Access
Research progress of stem cell therapy for neurological diseases
Muhammad Usman Taj1, Muhammad Asim2
1Department of Fisheries and Aquaculture, University of Veterinary and Animals Sciences, Lahore, Pakistan.
2Department of Zoology, University of Narowal, Narowal, Pakistan.
Correspondence: Muhammad Asim (Department of Zoology, University of Narowal, Narowal, Pakistan; E-mail: m.asim@uon.edu.pk).
Asia-Pacific Journal of Surgical & Experimental Pathology 2024, 1: 36-48. https://doi.org/10.32948/ajsep.2024.09.12
Received: 02 Sep 2024 | Accepted: 12 Sep 2024 | Published online: 17 Sep 2024
Key words mesenchymal stem cells, dental pulp stem cell, induced pluripotent stem cells, neurological diseases, stem cell transplantation
Therefore, curing neurological disorders is an urgent challenge for healthcare systems worldwide [3]. Neurological disorders include both peripheral and central nervous system disorders, with central nervous system disorders having a more complicated and challenging pathogenesis [3, 4]. Currently, there are few treatment options available for neurological disorders. Moreover, there are only a limited number of approved drugs on the market, and these drugs can slow the progression of the disease [5]. Several side effects can also occur when they are used [6]. Currently, there are significant therapeutic limitations in both surgical and pharmaceutical treatments for neurological illnesses. Stem cell research has been progressing steadily ever since the discovery of stem cells. Stem cell transplantation is increasingly being studied in relation to neurological diseases as a result of advances in cell therapy. Several major stem cell types such as embryonic stem cells (ESCs) and mesenchymal stem cells (MSCs) have demonstrated therapeutic potential and are strongly associated with cardiovascular and neurological diseases [7, 8]. Previous studies, such as Cebrian-Silla et al., surveyed cell populations in the mouse V-SVZ and found that neurons from the mouse SVZ migrated to the olfactory bulb [9]. Subsequently, new neurons were discovered in the mammalian brain[10], which was confirmed by further studies. Identification of these novel neurons has raised the possibility of using stem cells to treat neurological disorders.
Stem cells can release growth factors that aid in nerve regeneration. Consequently, many new clinical studies are now investigating how to regenerate neural tissue by modulating the innate immune response, reducing demyelination rates, or minimizing oxidative stress. Furthermore, given that some stem cells raise ethical and moral concerns when used, in this paper, we introduce the application of MSCs, dental pulp stem cells (DPSCs), and induced pluripotent stem cells (iPSCs) three types of stem cells with minimal ethical and moral implications. This will provide theoretical groundwork for the future treatment of neurological diseases.
There are various criteria for categorizing stem cells; in this context, classification is based on the different potentials of stem cells to more effectively utilize their differentiation capabilities.
1. Totipotent stem cells: which have the ability to self-renew and differentiate into any type of cell. For example, embryonic stem cells (ESCs), which exhibit morphological characteristics similar to those of early embryonic cells, have strong differentiation potential.
2. Pluripotent stem cells can differentiate into a variety of cell types but have a somewhat reduced capacity for differentiation compared to totipotent stem cells. They also have a relatively limited developmental capacity. Examples include mesenchymal stem cells (MSCs) and induced pluripotent stem cells (iPSCs).
3. Unipotent stem cells are found in the adult tissues and organs. These cells can differentiate only in a single direction, producing one specific type of cell. Examples include stem cells in the basal layer of epithelial tissues and myogenic stem cells in muscles, which exist in a stable state of self-renewal [11, 12]. This classification is showed in Figure 1.
Functions and properties of stem cells
Since their initial discovery in the 1860s, stem cells have been extensively researched and developed. The key characteristics of stem cells are as follows: (1) Self-renewal and differentiation potential: Stem cells are capable of continuous renewal and can differentiate into various types of cells or tissues. (2) Migration and homing abilities: Stem cells possess the ability to migrate to stem cell niches within different tissues and organs, where they perform specific activities based on the needs of the body [13]. (3) Low immunogenicity: Research has demonstrated that stem cells have low immunogenicity and exhibit "immune privilege" properties. They can stimulate specific immune cells that activate, proliferate, and differentiate, ultimately producing an immune response to antigens [14]. (4) Easily identifiable characteristics: Similar to MSCs, dental pulp stem cells (DPSCs) and stem cells from human exfoliated deciduous teeth (SHEDs) express MSC-related surface markers, such as leukocyte differentiation antigen and stromal cell antigen [15, 16]. (5) Secretion of active factors: Stem cells secrete growth factors, cytokines, and neurotrophic factors, which are biologically active molecules that regulate the metabolism and reproduction of tissue cells [17-19]. (6) Stem cell microenvironment: The stem cell microenvironment, also referred to as stem cell microniches, helps maintain the functional state of stem cells and prevents their excessive proliferation [20].
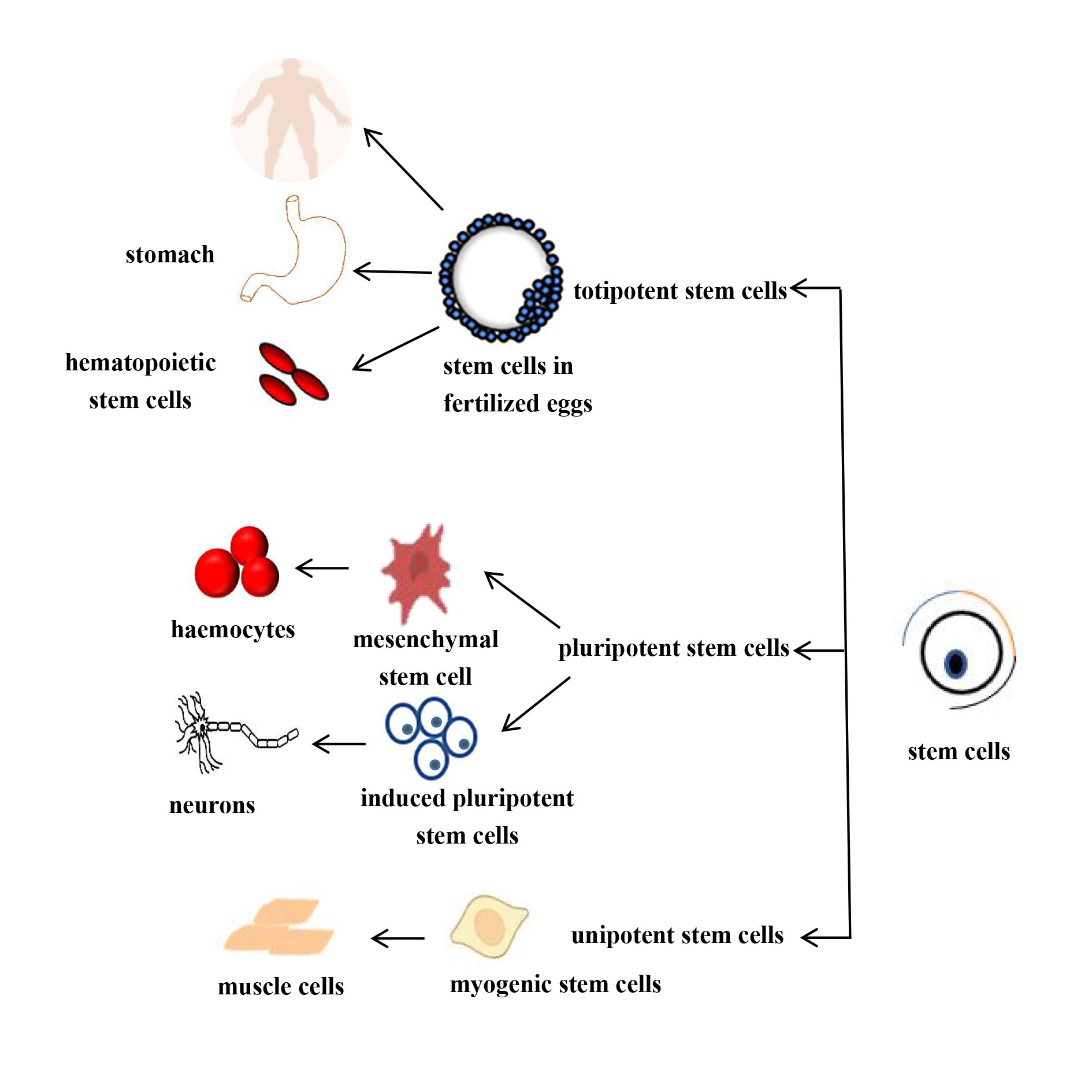 Figure 1. Schematic diagram of the differentiation potential of differentiating stem cells.
Figure 1. Schematic diagram of the differentiation potential of differentiating stem cells.
Neurological disorders are a group of diseases affecting the central and peripheral nervous systems. They can be categorized into three primary groups based on the pathophysiological mechanisms underlying their development: (1) disorders, including PD and AD, which are thought to be caused by the death of certain neurons or glial cells. (2) Acute damage causes nonspecific cell death (stroke or mechanical injury) in conditions such as traumatic brain injury (TBI) and traumatic spinal cord injury (SCI). (3) Diseases characterized by impaired nerve cell function, such as impaired neuromuscular junction (NMJ) function [22]. In summary, most diseases are caused by cell loss or death. On the other hand, stem cells have the ability to differentiate and secrete neurotrophic factors that promote cell growth and survival. Thus, stem cell therapy may have therapeutic potential for neurological disorders.
In recent years, stem cell therapy has expanded rapidly, and specialists in this field have conducted extensive studies on the application of stem cells in the treatment of neurological disorders. These findings suggest that stem cell therapy may be a viable treatment option for these conditions. To better illustrate the therapeutic potential of stem cells, we selected several types of stem cells that have demonstrated relatively good therapeutic efficacy in treating different neurological disorders for discussion and review purposes.
Mesenchymal stem cells
Mesenchymal stem cells (MSCs) were first proposed by Caplan et al. in the early 1990s and have since been found to perform various functions derived from different sources [23]. For instance, bone marrow mesenchymal stem cells (BM-MSCs) are promising candidates for the treatment of brain and spinal cord injuries. Similarly, adipose-derived mesenchymal stem cells hold potential for ovarian injury treatment and skin regeneration, whereas umbilical cord mesenchymal stem cells show promise for treating lung diseases and acute respiratory distress syndrome (ARDS) [23].
MSCs are a class of self-renewing, proliferating, and differentiating multipotent stem cells with immunoregulatory and neuroprotective properties. These cells can differentiate into specific mature cells such as nerve cells, bone cells, and cardiomyocytes in response to particular environmental stimuli. Additionally, MSCs secrete a variety of neurotrophic factors that promote the restoration of nervous system function and help mitigate the inflammatory response [24].
(1) Alzheimer's disease. Alzheimer's disease (AD) is the most common form of progressive neurodegenerative disease and the leading cause of dementia. It typically begins with mild cognitive impairment and short-term memory loss [25], and its pathogenesis is complex. It is well established that these symptoms are caused by degeneration of neurons in the hippocampus and cortex, which are essential for language, learning, and memory. Additionally, the deposition of amyloid-beta (Aβ) plaques is considered a hallmark of AD. Several treatment approaches for AD have been explored, including gene therapy, immunotherapy, peptidomimetic therapy, metal chelators, anti-amyloid, anti-tau, anti-neuroinflammatory, and neuroprotective drugs [26, 27]. However, these methods have significant limitations, such as difficulties in crossing the blood-brain barrier, challenges in identifying causative genes, and obstacles in performing gene editing, particularly in clinical trials. Stem cell therapy has significant advantages over other treatments, such as the ability of secreted exosomes to cross the blood-brain barrier. Stem cell therapy has been applied in a large number of animal models of AD. Indeed, the beneficial effects of extracellular vesicles from mesenchymal stem cells (MSCs) in modulating the inflammatory response have been reported after prolonged intravenous or intracerebroventricular administration in different mouse models of attention deficit disorder. Furthermore, Losurdo M showed for the first time that extracellular vesicles (EVs) from cytokine-pretreated MSCs, which can induce immunomodulatory and neuroprotective effects in AD, can be administered nasally. After reaching the brain, MSC-EVs reduce microglial activity and boost dendritic spine density [28]. BM-MSCs are a promising option to secrete anti-inflammatory and trophic factors and migrate to sites of inflammation and injury. For example, Redondo Castro et al. discovered that in vitro induction of BM-MSCs with IL-1 increases the expression of the trophic factor G-CSF through an IL-1 receptor type 1 (IL-1R1) mechanism and induces activated microglia to reduce the secretion of inflammatory mediators [29]. Additionally, Li et al. demonstrated that BM-MSCs not only modulate the expression of inflammatory factors, reducing the expression levels of IL-1 and IL-6, but also increase TGF-β levels, while simultaneously reducing oxidative stress and improving cognitive function. Although the aforementioned reports illustrate that MSCs can mediate inflammatory responses by regulating gene expression, their therapeutic effects diminish after in vitro expansion. To test this issue could be addressed, several researchers have cultured MSCs using tanshinone IIA (TIIA). According to Huang et al., tension-cultured mesenchymal stem cells (TIIA-MSCs) reduced the expression of interleukins (IL-1, IL-4, IL-6, and IL-10) and tumor necrosis factor (TNF-α) in rats, with IL-6 being the main distinction between the TIIA-MSC and MSCs groups. Additionally, TIIA-MSCs reduced the expression of Aβ generation-related mRNAs (BACE1 and PS1), marking the first time that TIIA-MSCs were found to be more neuroprotective than MSCs and offered better protection against the production of toxic proteins [30]. The findings of this study support the clinical use of MSCs, offering fresh hope for the treatment of AD, by indicating that the efficacy of MSCs is closely related to their regulation of Aβ production, which promotes the survival of hippocampal neurons by reducing BACE1 expression. Recently, as research has progressed, extracellular vesicles (EVs) derived from MSCs have also been found to be effective, as they exhibit immunoprotective and immunomodulatory capabilities similar to those of host MSCs, and have thus been identified as therapeutic candidates. According to Cone et al., mice treated with hMSC-EVs displayed cognitive abilities that were significantly higher than those of 5XFAD mice treated with saline [31]. This finding highlights the therapeutic benefits of extracellular vesicles and opens up a potential treatment option for AD. In addition to animal models, several experimental clinical studies have been conducted using MSCs. The results of a phase I clinical trial demonstrated the therapeutic potential of human umbilical cord blood mesenchymal stem cells injected into the lateral ventricles of individuals with AD dementia. Following this, MSCs were administered to nine patients with moderate-to-severe AD dementia via the right ventricle, showing their viability, safety, and tolerability. However, a transient febrile condition, lasting–1-2 days after each MSC injection was consistently observed [32]. Whether this symptom is related to the dose of the injected MSCs requires further investigation. Beyond AD, MSCs have also been found to stimulate regeneration pathways and promote axon reconstruction around ischemic lesions in various animal models of stroke [33]. Additionally, other types of stem cells have been explored in AD research (Table 1)., such as DPSCs [34], SHED [35], ESCs [36], and NSCs [37].
(2) Parkinson's disease. Parkinson's disease (PD) is caused by the degeneration of dopaminergic neurons in the substantia nigra of the brain. The primary symptoms of the disease include resting tremors, muscle stiffness, slowness of movement, and reduced ability to perform fine motor tasks [33]. PD is also accompanied by cognitive impairment as well as sleep and olfactory disturbances. Levodopa, along with methyldopa, is currently a widely used medication for the treatment of PD, both of which require long-term use. In addition to pharmacological treatment, adjuvant therapies and surgical deep brain stimulation (DBS) are also used to manage PD [38]. Although DBS has proven effective in some patients, it is an invasive surgical procedure, which excludes many patients from eligibility. Therefore, stem cell therapy for PD has gradually gained attention as a potential treatment option.Several investigations were first conducted using animal models to help prepare for clinical trials. First, behavioral testing using human BM-MSCs in a rat model of PD revealed a decrease in uncoordinated limb movements [39]. This led to a glimpse of the therapeutic potential of BM-MSCs. Subsequently, researchers questioned whether the efficacy of BM-MSCs could be related to the transplantation site. The next step was to directly inject BM-MSCs into the striatum in a rodent model of PD. This showed not only improved motor activity and enhanced neurogenesis but also induced neuroblast migration [40], which also indicates the importance of the stem cell transplantation modality. It has also been reported that MSC treatment inhibit synaptic nucleoproteins in PD models. Interestingly, Bcl2, an anti-apoptotic factor, was also upregulated. In PD mice, the expression of pro-apoptotic factors such as Bax and caspase 3 is reduced after MSC transplantation, suggesting a cytoprotective role in neurodegeneration [41]. PD may soon be cured if it is possible to specifically regulate the degeneration of dopaminergic neurons. In animal models of PD, MSCs are currently mostly supplied intracerebrally, but the therapeutic effect has not yet reached the optimal level. Recently, attempts to infuse medications intravenously, intra-arterially, and intensely have been documented in the literature. For example, after intravenous injection of adipose-derived mesenchymal stem cells (AD-MSCs), it was shown that AD-MSC treatment reduced astrocyte density and spatial memory impairment in PD can be improved [42]. In addition, intranasal administration of endometrial stem cells has been reported as a suitable treatment for PD. Intranasal injection can be used as a noninvasive method to improve PD symptoms in a dose-dependent manner in mouse models [43].
Animal studies have demonstrated that MSCs can affect PD symptoms, alter disease progression, and help control their manifestations. Subsequently, numerous experimental clinical studies have been conducted in various countries. Three techniques have been used to evaluate the outcomes of PD clinical trials: intracerebral stereotactic injections, systemic infusions, and intranasal infusions of BM-MSCs. Autologous BM-MSCs were injected into the subventricular zone of seven PD patients, and some experienced long-lasting improvements in motor function [44]. In another clinical trial, BM-MSCs were infused into the cerebral arteries of five patients with progressive supranuclear palsy, which shares some motor symptoms with PD. Unlike typical PD patients, these individuals generally experienced rapid declines in motor function, but clinical stability was observed for at least six months [45]. Additionally, patients with idiopathic PD who received allogeneic BM-MSCs or adipose tissue-derived mesenchymal cells showed positive changes in functional recovery, as well as improvements in facial expression and gait, according to a standard PD rating scale. MRI results also indicated enhanced functional connectivity between the striatum and substantia nigra [46]. Moreover, stem cell types other than MSCs have shown potential in aiding the development of dopaminergic neurons (Figure 2).
Dental pulp stem cells
Dental pulp stem cells (DPSCs), derived from the neural crest, are relatively easy to obtain and isolate [47]. To date, eight different types of stem cells have been identified [48] (Figure 3). DPSCs can be classified into two main categories: immature and mature. DPSCs express markers such as STRO-1, CD146, CD105, CD73, and CD90 [49]. Additionally, immature dental stem cells express embryonic stem cell (ESC) markers, such as OCT-4, Nanog, and SSEA-3, which aid in distinguishing between different stem cell types.
(1) Stroke. A blockage or rupture of a blood vessel in the brain can result in stroke, which is characterized by a disruption of the blood flow to a portion of the brain [50]. As a result, patients may experience motor, sensory, or speech deficits, as well as higher levels of brain dysfunction. These disorders require immediate medical care. For example, the tissue fibrinogen activator intravenous thrombolysis has a therapeutic time window and must be administered within 4.5 hours of the infarction's onset, or somewhat later if treated endovascularly, but not later than within 24 h [51]. Due to these time constraints, many patients miss the ideal time for therapy, making it urgently necessary to develop new treatment solutions to address this challenging issue. Ischemia-reperfusion is mostly used for the construction of animal models of stroke. In the current study, either ischemia was followed by intravenous injection of DPSCs or intracerebral injection was chosen in some studies. In experiments in which DPSCs were administered immediately after ischemia, after 3 or 4 hours, or even 24 hours, the therapeutic time window for DPSCs was explored. DPSCs were isolated from the impacted third molars of healthy volunteers and then intracranially injected 24 h post-ischemic stroke in Sprague Dawley rats that had been subjected to 2 h of middle cerebral artery occlusion [52]. DPSCs transplantation ameliorates neurological deficits and cerebral edema, reducing infarct size and decreasing the proportion of TUNEL-positive nuclei. In addition, it increases the number and proportion of NeuN-positive cells in the ischemic penumbra, increases the proportions of Bcl-2 and Bax, and down-regulates caspase 3 production in the cortical infarct areas. It also reduced the infarct volume and decreased the percentage of TUNEL-positive nuclei. Surprisingly, in all of these studies, there was a significant improvement in motor and cognitive function and a decrease in infarct volume in the DPSCs implantation group compared to the control group. In addition to the research mentioned above, the majority of investigations used DPSCs 24 hours after cerebral ischemia, regardless of the method of administration (intravenous or intracerebral). This suggests that DPSCs have a broad therapeutic window, providing optimism for patient care [53]. At 24 and 72 h after recovery, transplanted DPSCs significantly reduced infarct volume and reversed motor impairment. The infarct volume was dramatically reduced and motor function was significantly enhanced by DPSC transplantation 3 hours after reperfusion. Additionally, when compared to the control group, DPSC significantly reduced microglial activation, the expression of pro-inflammatory cytokines, and neuronal degeneration in the cortical ischemia border region [54]. Intriguingly, treatment with DPSCs restored forelimb extension in a rat model of stroke, and 28 days later, rats treated with stem cells displayed decreased immunolabeling for glial fibrillary acidic protein in tissue 1 mm from the infarct zone, suggesting a reduced proliferation of reactive astrocytes [55]. DPSC-derived exosomes can maintain neurovascular unit (NVU) integrity by regulating endogenous NG2 glial cell proliferation and differentiation, thereby reducing acute ischemic stroke (AIS)-induced injury and promoting repair. Research is ongoing, but it has been discovered that neural/glial antigen 2 (NG2)-expressing glial cells (NG2-glia) play a key role in regulating the (NVU) after AIS [56]. Previously, intracerebral or intraventricular administration was thought to be the most effective way to administer stem cell medications [54]. However, a recent study has shown that intravascular administration of DPSCs in a rodent model of focal cerebral ischemia reduced ischemic injury and improved motor function and proved to be the best way to treat stroke, especially when applied in the acute phase [51].
(2) Peripheral nerve injury. Peripheral nerve injury is one of the most common types of traumatic injuries to the nervous system. Although the peripheral nervous system has a greater capacity for regeneration compared to the central nervous system, several factors influence the degree of functional recovery after repair, primarily depending on the ability of Schwann cells to repair the injury [57]. Various treatment approaches have been explored depending on the type of injury. The gold standard for treating peripheral nerve injuries is nerve grafting and Schwann cell transplantation [58]. However, both procedures have limitations, such as limited availability of donor nerves, neuromuscular pain, donor site morbidity, and costs associated with secondary surgeries. If the differentiation capacity of stem cells can be utilized, this issue can be resolved. Takaoka et al. differentiated neural lineage cells (NLCs) from dental pulp stem cells (DPSCs) [59]. Additionally, NLCs were injected into immunocompromised rats with a 10 mm sciatic nerve defect to study the number of surviving cells and the level of differentiation in vivo. These findings demonstrated that NLCs increased endothelial cells, Schwann cells, and neuronal activity in a paracrine-dependent manner. Two weeks after transplantation, NLCs differentiated into platelet-derived growth factor receptor alpha (PDGFR) and oligodendrocyte progenitor cells (OPCs). After 12 weeks of observation, it was discovered that Schwann cell-like cells had survived and that there had been improvements in axonal development, remyelination, electrophysiological activity, and muscle atrophy [60]. The results of this work show how broadly applicable the DPSCs neural induction process is, and they also raise the possibility that NLCs created from human DPSCs could be a useful source for treating peripheral nerve damage. DPSCs can also work with biomaterials to accomplish various tasks. It has been reported that biomaterial nerve conduits embedded in DPSCs (polylactic acid-glycolic acid conduits) can promote the regeneration of damaged facial nerves [59]. Luo used a scaffold material synthesis scheme to create a third-generation neural regeneration catheter and selected a 10% GFD formulation (10% GelMA hydrogel, recombinant human basic fibroblast growth factor, and DPSCs) to fill cellulose/soy protein isolate composite membrane tubes [60]. This formed the CSM-GFD. Furthermore, a 15 mm long sciatic nerve defect in a rat model was repaired using a CSM-GFD catheter. The CSM-GFD catheter was found to regenerate neural tissue, such as neurons, Schwann nerve cells, and bone marrow stromal cells, at the histological level 12 weeks after implantation. Sciatic Nerve Function Index examination indicated that the CSM-GFD had recovered physically and functioned normally. In another study, differentiated DPSCs attached to a collagen scaffold demonstrated Schwann cell-associated characteristics and encouraged axon growth and myelin production in an in vitro model [61]. In addition to their ability to function independently, secreted neurotrophic factors have similar functions, including nerve growth factor, brain-derived neurotrophic factor, and glial cell line neurotrophic factor. These neurotrophic elements encourage the regeneration of peripheral nerves and offer defense against degeneration of facial motor neurons [62]. Axonal regeneration is aided by neurotrophic factors produced by cells derived from dental pulp and Schwann cells. In a mouse model, a tetracycline (Tet) induction system expressing the OLIG2 gene was used, which was then transfected into human DPSCs to encourage the repair and regeneration of injured peripheral nerves [63].
(3) Optic nerve injury. Photoreceptors, bipolar cells, and retinal ganglion cells (RGCs) constitute the retina, which is a component of the central nervous system [64]. Traumatic optic neuropathy (TON) can result from head injury, and chronic eye diseases, such as glaucoma, can also lead to a delayed loss of RGCs [65] and a corresponding decline in visual function [47]. Treatments for this condition include hyperbaric oxygen therapy, Chinese medicine (acupuncture, acupressure), and medications such as mannitol injections, burkholderia eye drops, and acetazolamide tablets. However, the ability to repair and regenerate retinal and optic nerve damage is limited by the presence of axonal growth inhibitory molecules and reduction of neurotrophic growth factors [66]. More studies are being conducted using stem cells to treat optic nerve injuries because of their ability to secrete various neurotrophic factors. First, DPSCs were injected into a rat model with an injured optic nerve, and the results demonstrated that DPSCs might support RGC survival and axonal regeneration through neurotrophin-mediated regulatory pathways [67]. In addition to DPSCs, BM-MSCs have also been investigated for the treatment of optic nerve injuries [68]. The results showed that hDPSC significantly promoted neuroprotection and neurogenesis in axotomized RGCs compared to hBMSC or MSC. It also identified VGF as a novel and potentially therapeutic hDPSC-derived neurotrophic factor (NTF) [69]. These findings suggest that DPSCs are a promising source for treating optic nerve injuries based on the treatment outcomes. Further studies found that intravitreal transplantation of DPSCs in an animal model of glaucoma preserved visual function for 35 days post-therapy and prevented RGC mortality [70]. However, its long-term efficacy in maintaining vision is limited, and re-infusion of stem cells or DPSCs could be considered to improve long-term outcomes. In the past, implanting primary photoreceptors partially restored visual function [71]. Since then, DPSCs have been shown to induce photoreceptor formation, and transplantation into the vitreous humor has been found to restore retinal nerve function and promote axonal regeneration [67]. After receiving FGF2 and SHH therapy, DPSCs develop into RGC and express high levels of neurotrophic factors, opening up the possibility of treating glaucoma [69]. More studies are being conducted using stem cells to treat optic nerve injury because they secrete a variety of neurotrophic factors. First, DPSCs were injected into a rat model of injured optic nerve, and the results demonstrated that DPSCs might support RGC survival and axonal regeneration through neurotrophin-mediated regulatory pathways [67]. BM-MSCs have also been investigated for optic nerve injury in addition to DPSCs [68]. The results showed that hDPSC significantly promoted neuroprotection and neurogenesis in axotomized RGCs compared to hBMSC or MSC. It also identified VGF as a novel and potentially therapeutic hDPSC-derived neurotrophic factor (NTF) [69]. This shows that DPSCs are a good source for treating optic nerve injuries based on the treatment outcomes. Another study found that intravitreal transplantation of DPSCs in an animal model of glaucoma preserved visual function for 35 days after therapy and prevented RGC mortality [70]. However, the efficacy of long-term maintenance of vision is poor, and reinfusion of stem cells or DPSCs could be considered for modification to enable long-term performance. Previously, implanting primary photoreceptors into the body partially restored visual function [71]. Since then, DPSCs have been shown that DPSCs can induce the formation of photoreceptors, and transplantation into the vitreous humor has been shown to restore function in retinal nerves and promote axonal regeneration [67]. After receiving FGF2 and SHH therapy, DPSCs develop into RGC and have a high level of NFT expression, opening the door to the possibility of treating glaucoma [69].
Induced pluripotent stem cells
Induced pluripotent stem cells (IPSCs) were successfully created from human fibroblasts in 2007 [72]. Since the discovery of these stem cells, their source of stem cells has been renewed (Figure 4). Since then, research on these cells has continued, revealing that IPSCs have differentiation potential similar that to of embryonic stem cells (ESCs), although there are some differences, as outlined in Table 2.
(1) Traumatic brain injury. Traumatic Brain Injury (TBI) is the leading cause of death and disability in children and adults. The lifetime of impaired cognition, motor function, and general quality of life may result from the severity of TBI, which is estimated to affect 64-74 million individuals worldwide annually [73]. It is now difficult to determine how to effectively treat patients with TBI and improve their quality of life. Currently, IPSCs cell-induced NSCs may be a promising strategy for stem cell replacement for brain injury in animal research trials [74]. For instance, Nieves et al. used NSCs derived from iPSCs transplantation to study the pathophysiology of male and female adult mice using a unilateral cortical contusion (CCI) model of sensorimotor brain injury [75]. Nieves et al. noted that the damaged host environment was better tolerated by NSCs compared to astrocytes. The NSCs and neuroblasts that survived gathered near the injection site in the corpus callosum below, as well as in the deep cortical layer. The outcomes of this study are intriguing and should demonstrate the possible use of stem cells. They used sensorimotor behavior tests and somatotopic estimates of host neurons, astrocytes, and microglia within the infected cortex to ascertain the effect of transplantation on neuropathology. The results revealed that the positive and negative effects of cell transplantation depend on the sex of the host, highlighting the significance of developing patient-specific therapeutic approaches for TBI. A recent study has shown that it is possible to follow the fate of IPSCs cell-induced NSCs in the host brain using in vivo MRI tracking techniques, such as the use of MEMRI by Jiang et al. to detect the neural activity induced by implanted IPSCs cells in local brain regions and to show the viability of this protocol [74]. This is true despite the promising outcomes of preclinical research. However, the existing in vitro models used to investigate TBI are insufficient because they do not faithfully imitate all elements and a variety of traumas. This is because of the pathogenic mechanisms of brain injury and its complexity. The advantage of using iPSCs technology is its ability to obtain specific cell types (neurons, astrocytes, and microglia) from healthy controls and people with a particular disease [76], which creates an ideal environment for screening the effects of targeted drugs. They also replicate brain development and illness more accurately than primary rodent cell cultures and immortalized human cancer cell lines. Additionally, by creating 2D and 3D model systems, iPSCs have the potential to create assays that are more complex and physiologically relevant [77]. In summary, iPSCs may have significant efficacy in the treatment of TBI.
(2) Spinal cord injury: The debilitating condition known as Spinal Cord Injury (SCI) affects the central nervous system and triggers several reactions, including ischemia, oxidative stress, inflammation, activation of apoptotic pathways, and motor dysfunction, which cause disability and multiple sequelae in patients [78, 79]. The common sequelae include paralysis and quadriplegia. Additionally, secondary SCI creates a microenvironment in the injured area that is unfavorable for neural regeneration following the structural damage caused by primary trauma. In the acute phase, current treatment options include maintaining the airway, breathing, and circulation or stabilizing the airway through early intubation along with spinal prophylaxis [80]. External spinal stabilization methods, such as avoidance of hypotension or use of cervical collars and backboards, are also employed to reduce dysfunction. Stem cell therapy has shown promising outcomes in the treatment of SCI. For instance, Lavoie recently used hiPSC-generated cells to treat moderate contusive SCI in adult immunodeficient rats by transplanting them into region-specific spinal cord neural progenitor cells (sNPCs). After 12 weeks, the transplanted sNPCs continued to grow and differentiate into neurons and glia that filled the lesion lumen and initiated an in vivo transcriptional program for the thoracic spinal cord. In addition, neurogenesis within the spinal cord tissue of neighboring hosts is promoted, resulting in the formation of synapses and myelin in host oligodendrocytes. Axons of transplanted hiPSC-derived sNPC cells extended cephalad and caudally from the SCI graft site, reaching the supraspinal regions approximately 6 cm cephalad. This finding suggests that iPSC-derived sNPCs can offer SCI patients a specific cell source that can act as a relay system at the site of injury [81]. Moreover, stem cell-derived exosomes have been identified as potential treatments for SCI. Exosomes from induced pluripotent stem cells (iPSCs-Exos) have been found to accelerate SCI recovery in LPS-treated bone marrow-derived macrophages (BMDMs), switch the polarization of M1 macrophages to the M2 phenotype, and enhance motor function in SCI mouse models in vivo [82]. Further investigation revealed that a functional participant in iPSCs-Exos is miR-199b-5p, whose overexpression induces M1 macrophage polarization to the M2 phenotype and promotes nerve regeneration in SCI. Rescue studies have shown that miR-199b-5p modulates cell growth factor (Hgf) and phosphatidylinositol 3-kinase (PI3K) signaling pathways to induce macrophage polarization and facilitate SCI recovery.
Additionally, iPSC-derived neural stem/progenitor cells were discovered to reduce remyelination [83], promote synaptogenesis and neurotrophic factor secretion [84], and improve functional recovery following SCI in a rat model. The risk of tumor formation is also being investigated [85]. Researchers have found that intrathecal implantation may yield better long-term effects, suggesting that different transplantation sites may result in varying outcomes [86]. Stem cell therapy has shown promising outcomes in the treatment of SCI. For instance, Lavoie recently used hiPSC-generated cells to treat moderately contusive SCI in adult immunodeficient rats by transplanting them into region-specific spinal cord neural progenitor cells (sNPC). After 12 weeks, transplanted sNPCs continued to grow and differentiate into neurons and glia that filled the lesion lumen and produced an in vivo transcriptional program for the thoracic spinal cord. In addition, neurogenesis within the spinal cord tissue of neighboring hosts is promoted, resulting in the formation of synapses and myelin in host oligodendrocytes. Axons of transplanted hiPSCs from sNPC-derived cells extend cephalad and caudally from the SCI graft site, reaching the supraspinal regions approximately 6 cm cephalad. This finding suggests that iPSC-derived sNPC can offer SCI patients a cell source that can act as a relay system at the site of injury [81]. In addition, stem cell-derived exosomes have been identified as potential treatments for SCI. Exosomes from induced pluripotent stem cells (iPSCs-Exo) have been shown to accelerate SCI recovery in LPS-treated bone marrow-derived macrophages (BMDM), change the polarization of M1 macrophages to the M2 phenotype, and enhance motor function in SCI mouse models in vivo [82]. A functional participant of iPSCs-Exos was discovered through further investigation to be miR-199b-5p, whose overexpression induced M1 macrophage polarization to the M2 phenotype and promoted nerve regeneration in SCI. Rescue studies have shown that miR-199b-5p modulates cell growth factor (Hgf) and phosphatidylinositol 3-kinase (PI3K) signaling pathways to induce macrophage polarization and SCI recovery. Additionally, IPSC-derived neural stem/progenitor cells were discovered to reduce remyelination [83], promote synaptogenesis and neurotrophic factor secretion [84], and improve functional recovery following SCI in a rat model. The risk of tumor formation has also been studied [85]. Researchers have discovered that intrathecal implantation may yield better long-term effects, demonstrating that different locations of transplantation may result in different outcomes [86].
|
Table 1. Effectiveness of different types of stem cells applied to AD. |
|||
|
Stem Cell Types |
Advantages |
Functions |
References |
|
Easy access to materials and multipotential |
Inhibition of Tau protein phosphorylation Improves cell viability Reduction of apoptosis Protection of microtubules |
[34] |
|
|
SHED |
Simple access to resources and potential |
Improves cell viability
|
[35] |
|
ESCs |
Unlimited self-renewal ability |
Improved cognitive ability in mice |
[36] |
|
NSCs |
Differentiation in direction
|
Improved spatial memory impairment in model mice |
[37] |
|
DPSCS: dental pulp stem cells;SHED: deciduous milk tooth stem cells; ESCs: embryonal stem cell; NSCs: neural stem cell. |
|||
|
Table 2. Differences between IPSCs and ESCs. |
||
|
Items |
IPSCs |
ESCs |
|
Features |
Can be obtained from adult autologous somatic cells |
Can only be derived from early blastocysts, which has limitations |
|
No allogeneic immuno-matching issues |
Risk of tumor formation |
|
|
Ethical and moral issues involved |
Ethical and moral issues involved |
|
|
More suitable for clinical applications |
Banned by most countries |
|
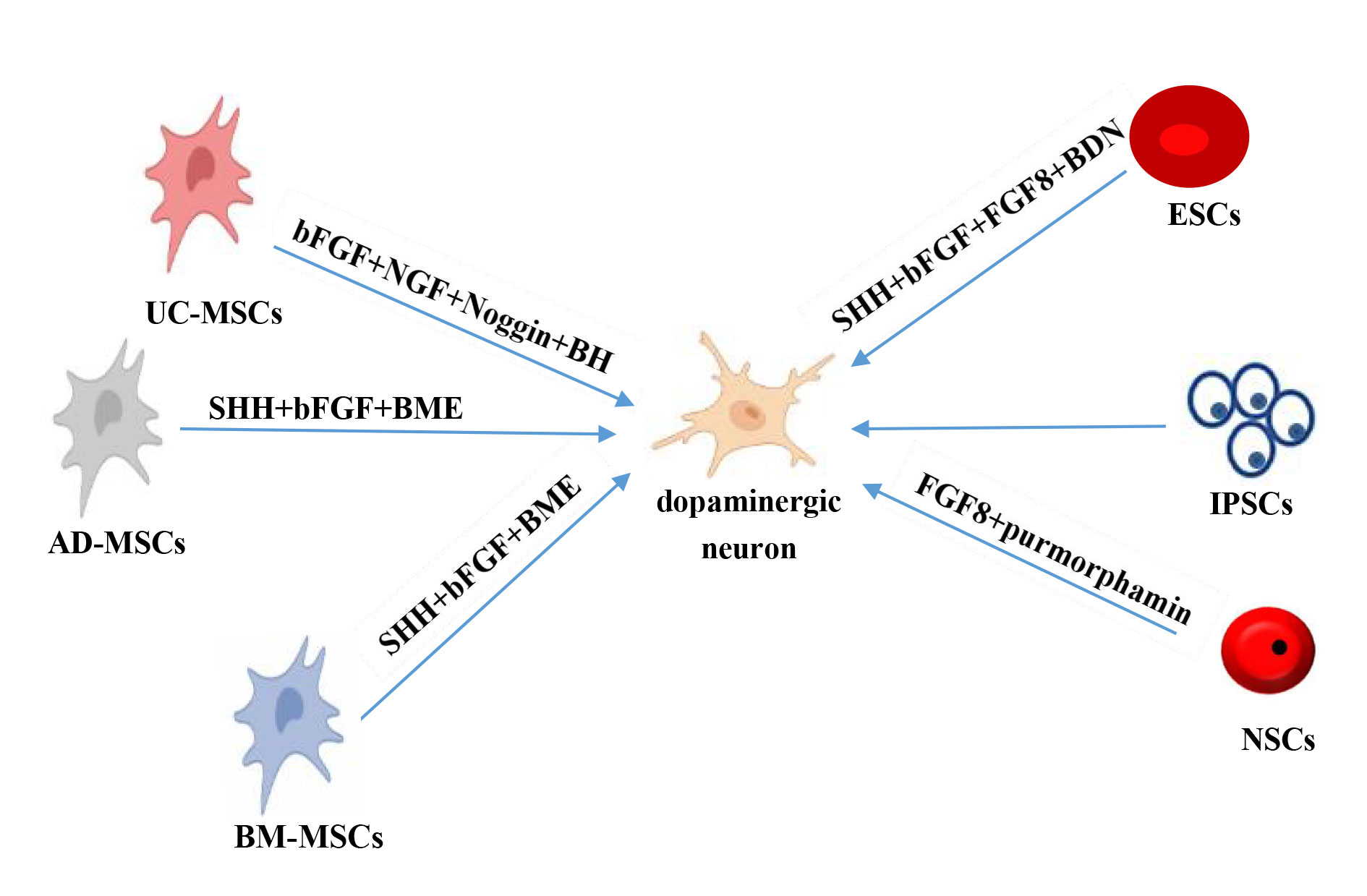 Figure 2. Differentiation of different types of stem cells into dopaminergic neurons. AD- MSCs: adipose tissue-derived mesenchymal stem cells; BM- MSCs: bone marrow-derived mesenchymal stem cells; NSCs: neural stem cells; UC-MSCs: umbilical cord-derived mesenchymal stem cells; bFGF: basic fibroblast growth factor; BME: b-Mercaptoethanol; BDNF: Brain-derived neurotrophic factor; FGF8: fibroblast growth factor 8; NGF: nerve growth factor; SHH: sonic hedgehog.
Figure 2. Differentiation of different types of stem cells into dopaminergic neurons. AD- MSCs: adipose tissue-derived mesenchymal stem cells; BM- MSCs: bone marrow-derived mesenchymal stem cells; NSCs: neural stem cells; UC-MSCs: umbilical cord-derived mesenchymal stem cells; bFGF: basic fibroblast growth factor; BME: b-Mercaptoethanol; BDNF: Brain-derived neurotrophic factor; FGF8: fibroblast growth factor 8; NGF: nerve growth factor; SHH: sonic hedgehog.
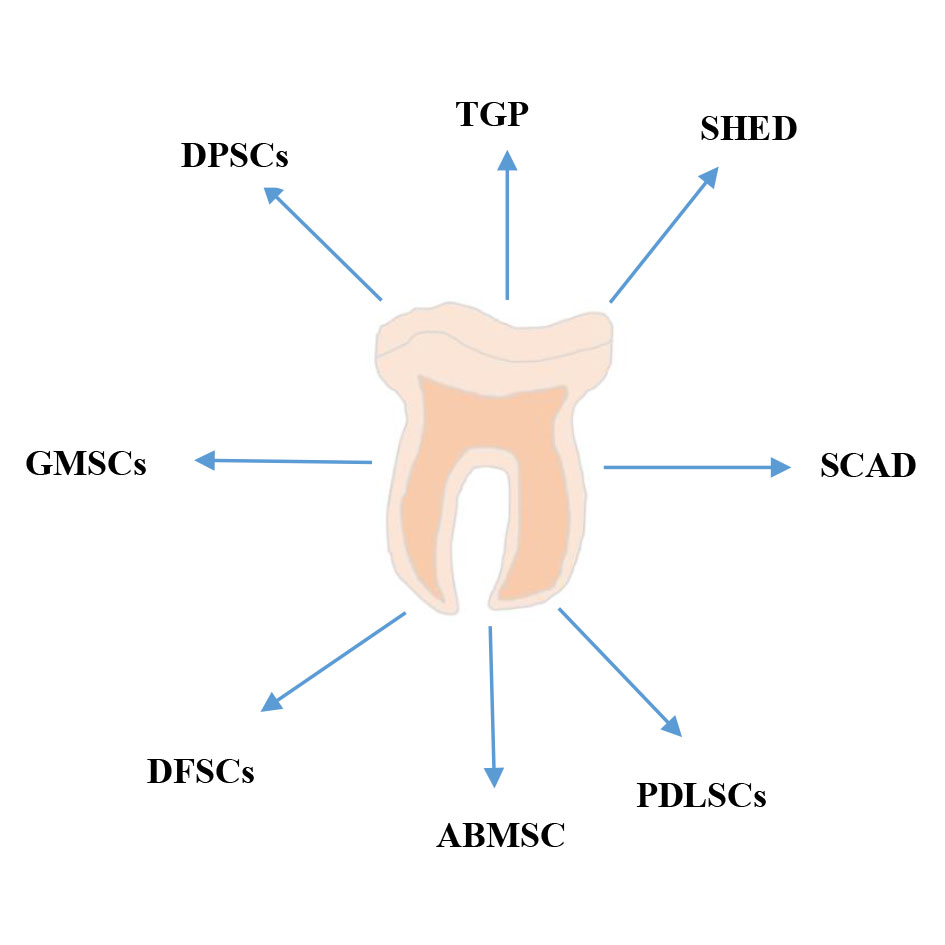 Figure 3. Origin of different types of dental pulp cells. DPSCs: dental pulp stem cells; GMSCs: gingival mesenchymal stem cells; DFSCs: dental capsule stem cells; ABMSCs: alveolar mesenchymal stem cells; PDLSCs: periodontal stem cells; SCAD: apical milk tooth stem cells; SHED: deciduous milk tooth stem cells; TGPC: dental germ stem cells.
Figure 3. Origin of different types of dental pulp cells. DPSCs: dental pulp stem cells; GMSCs: gingival mesenchymal stem cells; DFSCs: dental capsule stem cells; ABMSCs: alveolar mesenchymal stem cells; PDLSCs: periodontal stem cells; SCAD: apical milk tooth stem cells; SHED: deciduous milk tooth stem cells; TGPC: dental germ stem cells.
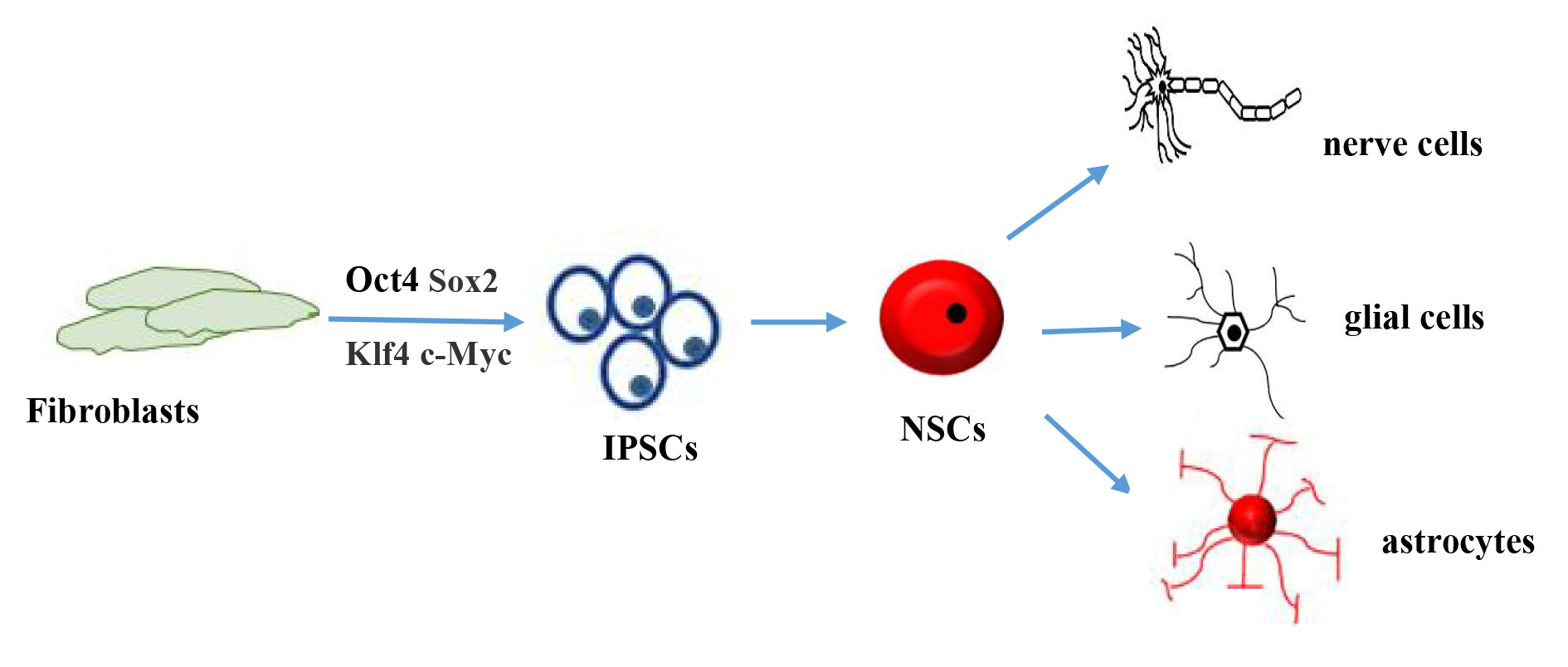 Figure 4. Schematic diagram of induced pluripotent stem cell differentiation. Fibroblasts are transformed into iPSCs in the presence of four inducing factors: Oct4, Sox2, Klf4, and c-Myc. Thereafter, they can be transformed into neural stem cells under certain conditions and differentiate into other cell types such as neurons.
Figure 4. Schematic diagram of induced pluripotent stem cell differentiation. Fibroblasts are transformed into iPSCs in the presence of four inducing factors: Oct4, Sox2, Klf4, and c-Myc. Thereafter, they can be transformed into neural stem cells under certain conditions and differentiate into other cell types such as neurons.
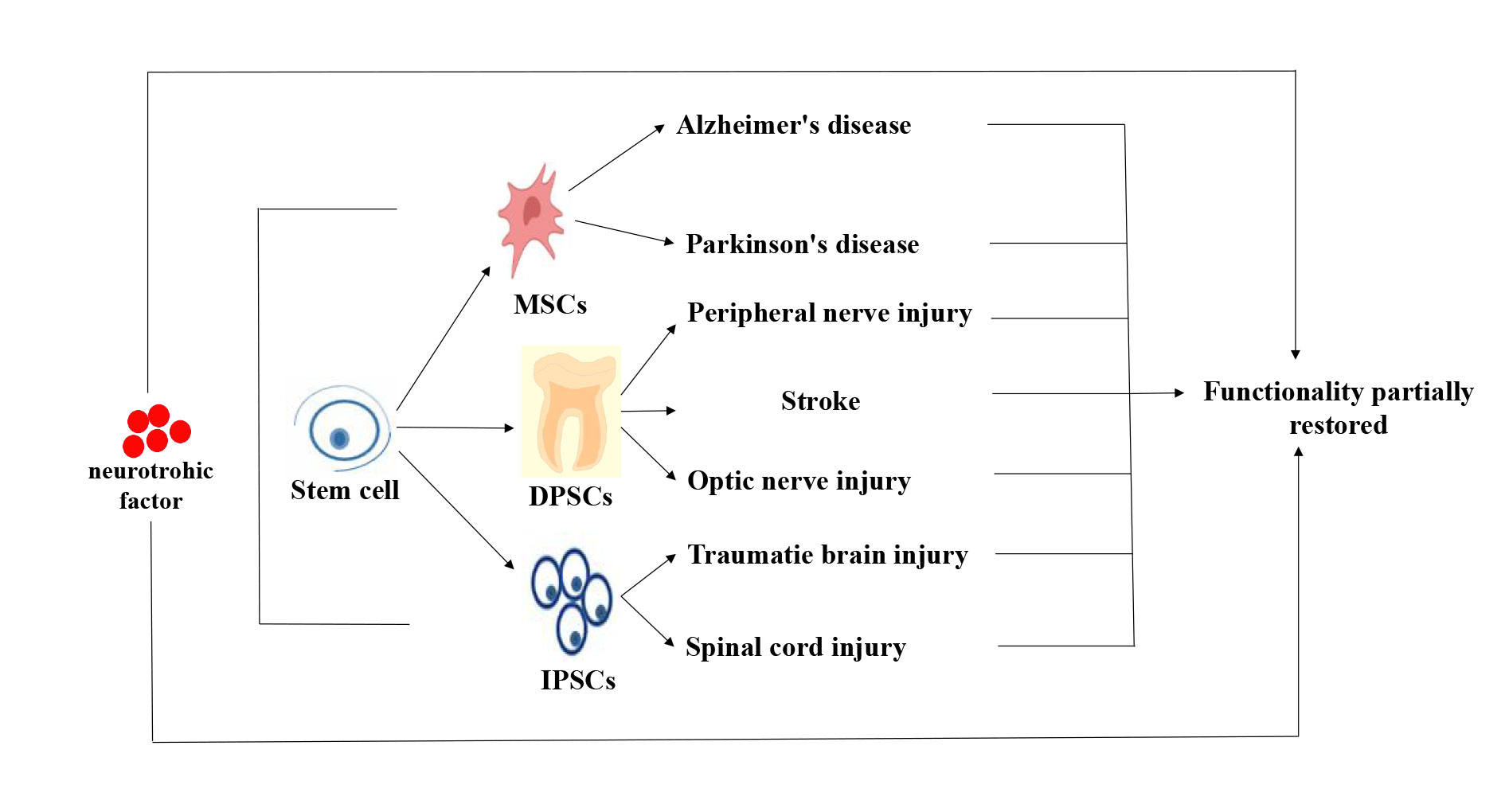 Figure 5. Stem cell therapy for neurological disorders. Neurological disorders were partially improved or restored after stem cell therapy. At the same time, the secretion of neurotrophic factors can also improve their functions.
Figure 5. Stem cell therapy for neurological disorders. Neurological disorders were partially improved or restored after stem cell therapy. At the same time, the secretion of neurotrophic factors can also improve their functions.
Surgery, along with neurotrophic and rehabilitative training, is a common treatment option. Currently, stem cell therapy for neurotraumatic diseases has achieved relatively positive results, improving the therapeutic effect of stem cells and reducing the risk of transplantation [87, 88], and the specific mechanism of action of stem cells is the focus of subsequent research.
First, ethical and moral issues are faced when it comes to the application of stem cells. Second, the heterogeneity of stem cells must be considered. For example, BM-MSCs are a mixed population of cells with different cell subpopulations, some of which have multipotent properties and some of which have potential properties [89]. Common side effects of BM-MSC injections are usually rare, with the most common being low or moderate fever; however, body temperatures rarely exceed 38°C. A small percentage of patients may also experience a minor headache following the injection, occasionally along with nausea and vomiting, which usually disappears in about half a day. After receiving an MSC injection, a small percentage of patients may experience facial flushing, although this will eventually go away. Finally, MSCs often suffer from senescence and decreased differentiation capacity, limiting their widespread application [90]. 3D culture techniques can be combined to maximize the stem cell potential.
Compared to BM-MSC, DPSCs have a higher self-proliferative and immunomodulatory capacity and are easier to access with fewer ethical issues. These characteristics make DPSCs an ideal source of stem cells for regenerative medicine and disease treatments. However, as was already indicated, there are certain variations between DPSC and SHED in terms of associated factor expression, differentiation, and proliferation. Therefore, an additional analysis of cellular characteristics was performed to sort particular cell groups.
Informed consent is a major ethical issue in the derivation and application of IPSCs, and IPSCs can only be induced from somatic cells if the cell donor consents to the removal of the cells from their body for IPSC derivation [91]. Currently, the method by which transcription factors cause cells to become iPSCs is poorly understood, and the success rate of reprogramming is low. When differentiated cells are transplanted into the body, the method of adding four "reprogramming" genes or replacing defective genes in sick cells has the potential to cause cancer. Additionally, serious ethical problems arise when transplanted differentiated cells cause unchecked cell proliferation and tumor growth at the site of implantation.
Although iPSCs are capable of differentiation, proliferation, and development, IPSCs are genetically and epigenetically less stable because of their propensity to change cell karyotypes, fast proliferation, and high differentiation potential. In addition, whether iPSCs are the same as human ESCs in terms of their biological properties and how they recognize and remove cancer cells during induced differentiation needs to be further investigated. Differentiating iPSCs into specific cell types and delivering differentiated cells safely and effectively into the body are key considerations. Most importantly, current stem cell therapy is expensive and unaffordable for the average family. Therefore, the safety and reliability assessment system for clinical applications needs to be improved.
It takes time to go from cell access to transplantation, and shortening that time also needs to be considered [92]. The biological activity of stem cells in the body decreases with age. One of the best sources of stem cells has been discovered to be DPSCs, which are also simple to harvest. Because of this, stem cells can be grown from it to create a personal cell bank. In addition, stem cells can be transplanted in a variety of ways, and it is important to determine which neurological disorders are best served by different transplantation methods [33]. Simultaneously, the blood-brain barrier prevents drug molecules from reaching the site of cure. However, the study discovered that exosomes could penetrate the blood-brain barrier, whether or not the drug molecules could be wrapped in them, which will be utilized as a carrier of drug molecules.
Among the challenges facing drug developers of neurodegenerative disease therapies are that symptoms are often complex and that animal models do not fully recapitulate the unique features of the human nervous system. However, the technology of human iPSCs will allow us to generate neuronal cells from a single patient, thus eliminating the species specificity problem inherent when using animal models. A strong link between AD and the apolipoprotein E (APOE) gene has now been found [93, 94]. The use of iPSCs to construct cell lines with different APOEs has improved our understanding of AD pathogenesis. In addition, Chang et al. found that iPSCs from AD patients with genetic mutations was able to recapitulate the cellular features of AD, and this cellular model was constructed to provide a suitable pathological model for drug screening [95]. Currently, iPSCs have been widely used for modeling heart diseases, studying genetic arrhythmias, neurological diseases, and many other disease models, which provides a possible way to develop and exploit new drugs [96]. If iPSCs technology can be utilized to develop new drugs and screen effective drug classes, it will be a big step forward for the treatment of neurological diseases.
In summary, we can draw the following conclusions: stem cells have the potential to treat neurological diseases, and it is hoped that they will soon be used in clinical settings.
No applicable.
Ethics approval
No applicable.
Data availability
The Data will be available upon request.
Funding
The authors did not receive any financial support from any organization for the submitted work.
Authors’ contribution
MUT and MA both significantly contributed to the conception, design, and writing of this review article. MA read and approved the final version of the manuscript.
Competing interests
The authors have diligently stated that they have no conflicts of interest to report.
- Feigin VL, Vos T, Nichols E, Owolabi MO, Carroll WM, Dichgans M, Deuschl G, Parmar P, Brainin M, Murray C: The global burden of neurological disorders: translating evidence into policy. Lancet Neurol 2020, 19(3): 255-265.
- El Gaamouch F, Chen F, Ho L, Lin HY, Yuan C, Wong J, Wang J: Benefits of dietary polyphenols in Alzheimer's disease. Front Aging Neurosci 2022, 14: 1019942.
- Alessandrini M, Preynat-Seauve O, De Bruin K, Pepper MS: Stem cell therapy for neurological disorders. S Afr Med J 2019, 109(8b):70-77.
- Wang D, Wang Y, Tian W, Pan J: Advances of tooth-derived stem cells in neural diseases treatments and nerve tissue regeneration. Cell Prolif 2019, 52(3): e12572.
- Choong CJ, Baba K, Mochizuki H: Gene Therapy for Neurological Disorders. Expert Opin Biol Ther 2016, 16(2): 143-159.
- Friedman D, French JA, Maccarrone M: Safety, efficacy, and mechanisms of action of cannabinoids in neurological disorders. Lancet Neurol 2019, 18(5): 504-512.
- Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z: Stem cells: past, present, and future. BioMed Central 2019, 10(1): 68.
- Hernández R, Jiménez-Luna C, Perales-Adán J, Perazzoli G, Melguizo C, et al.: Differentiation of human mesenchymal stem cells towards Neuronal Lineage: Clinical Trials in Nervous System Disorders. Biomol Ther (Seoul) 2020, 28(1): 34-44.
- Cebrian-Silla A, Nascimento MA, Redmond SA, Mansky B, Wu D, Obernier K, Romero Rodriguez R, Gonzalez-Granero S, García-Verdugo JM, Lim DA et al: Single-cell analysis of the ventricular-subventricular zone reveals signatures of dorsal and ventral adult neurogenesis. Elife 2021, 10: e67436.
- Libbrecht S, Van den Haute C, Welkenhuysen M, Braeken D, Haesler S, Baekelandt V: Chronic chemogenetic stimulation of the anterior olfactory nucleus reduces newborn neuron survival in the adult mouse olfactory bulb. J Neurochem 2021, 158(5): 1186-1198.
- Alatyyat SM, Alasmari HM, Aleid OA, Abdel-Maksoud MS, Elsherbiny N: Umbilical cord stem cells: Background, processing and applications. Tissue Cell 2020, 65: 101351.
- Gancheva M, Kremer K, Gronthos S, Koblar S: Using Dental Pulp Stem Cells for Stroke Therapy. Frontiers in neurology 2019, 10: 422.
- Naji A, Eitoku M, Favier B, Deschaseaux F, Rouas-Freiss N, Suganuma N: Biological functions of mesenchymal stem cells and clinical implications. Cellular and molecular life sciences : CMLS 2019, 76(17): 3323-3348.
- Tang Y, Yu P, Cheng L: Current progress in the derivation and therapeutic application of neural stem cells. Cell death & disease 2017, 8(10): e3108.
- Granados-Montiel J, Cruz-Lemini M, Rangel-Escareño C, Martinez-Nava G, Landa-Solis C, Gomez-Garcia R, Lopez-Reyes A, Espinosa-Gutierrez A, Ibarra C: SERPINA9 and : Novel Cartilage Lineage Differentiation Markers of Human Mesenchymal Stem Cells with Kartogenin. Cartilage 2021, 12(1): 102-111.
- Wang D, Wang Y, Tian W, Pan J: Advances of tooth-derived stem cells in neural diseases treatments and nerve tissue regeneration. Cell proliferation 2019, 52(3): e12572.
- Ding C, Zou Q, Wang F, Wu H, Chen R, Lv J, Ling M, Sun J, Wang W, Li H et al: Human amniotic mesenchymal stem cells improve ovarian function in natural aging through secreting hepatocyte growth factor and epidermal growth factor. Stem Cell Res Ther 2018, 9(1): 55.
- Zhang Z, Wang Y, Li M, Li J, Wu J: Fibroblast growth factor 18 increases the trophic effects of bone marrow mesenchymal stem cells on chondrocytes isolated from late stage osteoarthritic patients. Stem Cells Int 2014, 2014: 125683.
- Pollock K, Dahlenburg H, Nelson H, Fink KD, Cary W, Hendrix K, Annett G, Torrest A, Deng P, Gutierrez J et al: Human Mesenchymal Stem Cells Genetically Engineered to Overexpress Brain-derived Neurotrophic Factor Improve Outcomes in Huntington's Disease Mouse Models. Mol Ther 2016, 24(5): 965-977.
- Wu C, Xue L, Su L, Xie J, Jiang H, Yu X, Liu H: Magnesium promotes the viability and induces differentiation of neural stem cells both in vitro and in vivo. Neurological research 2019, 41(3): 208-215.
- Soria Lopez JA, González HM, Léger GC: Alzheimer's disease. Handb Clin Neurol 2019, 167: 231-255.
- Isaković J, Šerer K, Barišić B, Mitrečić D: Mesenchymal stem cell therapy for neurological disorders: The light or the dark side of the force? Front Bioeng Biotechnol 2023, 11: 1139359.
- Hoang DM, Pham PT, Bach TQ, Ngo ATL, Nguyen QT, Phan TTK, Nguyen GH, Le PTT, Hoang VT, Forsyth NR et al: Stem cell-based therapy for human diseases. Signal Transduct Target Ther 2022, 7(1): 272.
- Chung JW, Chang WH, Bang OY, Moon GJ, Kim SJ, Kim SK, Lee JS, Sohn SI, Kim YH: Efficacy and Safety of Intravenous Mesenchymal Stem Cells for Ischemic Stroke. Neurology 2021, 96(7): e1012-e1023.
- Lane CA, Hardy J, Schott JM: Alzheimer's disease. Eur J Neurol 2018, 25(1): 59-70.
- Khan S, Barve KH, Kumar MS: Recent Advancements in Pathogenesis, Diagnostics and Treatment of Alzheimer's Disease. Curr Neuropharmacol 2020, 18(11): 1106-1125.
- Yu TW, Lane HY, Lin CH: Novel Therapeutic Approaches for Alzheimer's Disease: An Updated Review. Int J Mol Sci 2021, 22(15): 8208.
- Losurdo M, Pedrazzoli M, D'Agostino C, Elia CA, Massenzio F, Lonati E, Mauri M, Rizzi L, Molteni L, Bresciani E et al: Intranasal delivery of mesenchymal stem cell-derived extracellular vesicles exerts immunomodulatory and neuroprotective effects in a 3xTg model of Alzheimer's disease. Stem Cells Transl Med 2020, 9(9): 1068-1084.
- Redondo-Castro E, Cunningham C, Miller J, Martuscelli L, Aoulad-Ali S, Rothwell NJ, Kielty CM, Allan SM, Pinteaux E: Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Res Ther 2017, 8(1): 79.
- Huang N, Li Y, Zhou Y, Zhou Y, Feng F, Shi S, Ba Z, Luo Y: Neuroprotective effect of tanshinone IIA-incubated mesenchymal stem cells on Aβ(25-35)-induced neuroinflammation. Behav Brain Res 2019, 365: 48-55.
- Cone AS, Yuan X, Sun L, Duke LC, Vreones MP, Carrier AN, Kenyon SM, Carver SR, Benthem SD, Stimmell AC et al: Mesenchymal stem cell-derived extracellular vesicles ameliorate Alzheimer's disease-like phenotypes in a preclinical mouse model. Theranostics 2021, 11(17): 8129-8142.
- Kim HJ, Cho KR, Jang H, Lee NK, Jung YH, Kim JP, Lee JI, Chang JW, Park S, Kim ST et al: Intracerebroventricular injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer's disease dementia: a phase I clinical trial. Alzheimers Res Ther 2021, 13(1): 154.
- Andrzejewska A, Dabrowska S, Lukomska B, Janowski M: Mesenchymal Stem Cells for Neurological Disorders. Adv Sci (Weinh) 2021, 8(7):2002944.
- Luo L, He Y, Wang X, Key B, Lee BH, Li H, Ye Q: Potential Roles of Dental Pulp Stem Cells in Neural Regeneration and Repair. Stem Cells Int 2018, 2018: 1731289.
- Nicola F, Marques MR, Odorcyk F, Petenuzzo L, Aristimunha D, Vizuete A, Sanches EF, Pereira DP, Maurmann N, Gonçalves CA et al: Stem Cells from Human Exfoliated Deciduous Teeth Modulate Early Astrocyte Response after Spinal Cord Contusion. Mol Neurobiol 2019, 56(1): 748-760.
- Sun Y, Hong F, Zhang L, Feng L: The sphingosine-1-phosphate analogue, FTY-720, promotes the proliferation of embryonic neural stem cells, enhances hippocampal neurogenesis and learning and memory abilities in adult mice. Br J Pharmacol 2016, 173(18): 2793-2807.
- Hayashi Y, Lin HT, Lee CC, Tsai KJ: Effects of neural stem cell transplantation in Alzheimer's disease models. J Biomed Sci 2020, 27(1): 29.
- Gao C, Liu J, Tan Y, Chen S: Freezing of gait in Parkinson's disease: pathophysiology, risk factors and treatments. Transl Neurodegener 2020, 9: 12.
- Meligy FY, Elgamal DA, Abd Allah ESH, Idriss NK, Ghandour NM, Bayoumy EMR, Khalil ASA, El Fiky MM, Elkhashab M: Testing alternatives: the use of adipose-derived mesenchymal stem cells to slow neurodegeneration in a rat model of Parkinson's disease. Mol Biol Rep 2019, 46(6): 5841-5858.
- Chen D, Fu W, Zhuang W, Lv C, Li F, Wang X: Therapeutic effects of intranigral transplantation of mesenchymal stem cells in rat models of Parkinson's disease. J Neurosci Res 2017, 95(3): 907-917.
- Wang YL, Liu XS, Wang SS, Xue P, Zeng ZL, Yang XP, Zhang SM, Zheng W, Hua L, Li JF et al: Curcumin-Activated Mesenchymal Stem Cells Derived from Human Umbilical Cord and Their Effects on MPTP-Mouse Model of Parkinson's Disease: A New Biological Therapy for Parkinson's Disease. Stem Cells Int 2020, 2020: 4636397.
- Hamedi H, Ghorbanian SH, Mirzaeian L, Abrari K, Mozdziak P, Ghorbanian MT: Intravenous Transplantation of Adipose-Derived Mesenchymal Stem Cells Promoted The Production of Dopaminergic Neurons and Improved Spatial Memory in A Rat Model of Parkinson's Disease. Cell J 2023, 25(5): 317-326.
- Bagheri-Mohammadi S, Alani B, Karimian M, Moradian-Tehrani R, Noureddini M: Intranasal administration of endometrial mesenchymal stem cells as a suitable approach for Parkinson's disease therapy. Mol Biol Rep 2019, 46(4): 4293-4302.
- Venkataramana NK, Kumar SK, Balaraju S, Radhakrishnan RC, Bansal A, Dixit A, Rao DK, Das M, Jan M, Gupta PK et al: Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson's disease. Transl Res 2010, 155(2): 62-70.
- Canesi M, Giordano R, Lazzari L, Isalberti M, Isaias IU, Benti R, Rampini P, Marotta G, Colombo A, Cereda E et al: Finding a new therapeutic approach for no-option Parkinsonisms: mesenchymal stromal cells for progressive supranuclear palsy. J Transl Med 2016, 14(1): 127.
- Chen Y, Shen J, Ke K, Gu X: Clinical potential and current progress of mesenchymal stem cells for Parkinson's disease: a systematic review. Neurol Sci 2020, 41(5): 1051-1061.
- Mead B, Logan A, Berry M, Leadbeater W, Scheven BA: Concise Review: Dental Pulp Stem Cells: A Novel Cell Therapy for Retinal and Central Nervous System Repair. Stem Cells 2017, 35(1): 61-67.
- Lei T, Zhang X, Du H: Characteristics, Classification, and Application of Stem Cells Derived from Human Teeth. Stem Cells Int 2021, 2021: 8886854.
- Luo L, Wang X, Zhang Y, Wu Y, Hu F, Xing Z, Wang L, Xiao J, Guastaldi F, He Y et al: Biological Behavioral Alterations of the Post-neural Differentiated Dental Pulp Stem Cells Through an in situ Microenvironment. Front Cell Dev Biol 2020, 8: 625151.
- Pan Z, Ma G, Kong L, Du G: Hypoxia-inducible factor-1: Regulatory mechanisms and drug development in stroke. Pharmacol Res 2021, 170: 105742.
- Nito C, Suda S, Nitahara-Kasahara Y, Okada T, Kimura K: Dental-Pulp Stem Cells as a Therapeutic Strategy for Ischemic Stroke. Biomedicines 2022, 10(4): 734.
- Gong P, Tian Q, He Y, He P, Wang J, Guo Y, Ye Q, Li M: Dental pulp stem cell transplantation facilitates neuronal neuroprotection following cerebral ischemic stroke. Biomed Pharmacother 2022, 152: 113234.
- Wu T, Xu W, Chen H, Li S, Dou R, Shen H, Liu X, Liu X, Hong Y, He J: Comparison of the differentiation of dental pulp stem cells and periodontal ligament stem cells into neuron-like cells and their effects on focal cerebral ischemia. Acta Biochim Biophys Sin (Shanghai) 2020, 52(9): 1016-1029.
- Nito C, Sowa K, Nakajima M, Sakamoto Y, Suda S, Nishiyama Y, Nakamura-Takahashi A, Nitahara-Kasahara Y, Ueda M, Okada T et al: Transplantation of human dental pulp stem cells ameliorates brain damage following acute cerebral ischemia. Biomed Pharmacother 2018, 108: 1005-1014.
- Yew WP, Djukic ND, Jayaseelan JSP, Kaidonis X, Kremer KL, Choy FC, Woodman RJ, Koblar SA, Sims NR: Delayed Treatment with Human Dental Pulp Stem Cells Accelerates Functional Recovery and Modifies Responses of Peri-Infarct Astrocytes Following Photothrombotic Stroke in Rats. Cell Transplant 2021, 30: 963689720984437.
- Hu X, Geng P, Zhao X, Wang Q, Liu C, Guo C, Dong W, Jin X: The NG2-glia is a potential target to maintain the integrity of neurovascular unit after acute ischemic stroke. Neurobiol Dis 2023, 180: 106076.
- Bosch-Queralt M, Fledrich R, Stassart RM: Schwann cell functions in peripheral nerve development and repair. Neurobiol Dis 2023, 176: 105952.
- Tamaki T, Hirata M, Nakajima N, Saito K, Hashimoto H, Soeda S, Uchiyama Y, Watanabe M: A Long-Gap Peripheral Nerve Injury Therapy Using Human Skeletal Muscle-Derived Stem Cells (Sk-SCs): An Achievement of Significant Morphological, Numerical and Functional Recovery. PLoS One 2016, 11(11): e0166639.
- Takaoka S, Uchida F, Ishikawa H, Toyomura J, Ohyama A, Watanabe M, Matsumura H, Marushima A, Iizumi S, Fukuzawa S et al: Transplanted neural lineage cells derived from dental pulp stem cells promote peripheral nerve regeneration. Hum Cell 2022, 35(2): 462-471.
- Luo L, He Y, Jin L, Zhang Y, Guastaldi FP, Albashari AA, Hu F, Wang X, Wang L, Xiao J et al: Application of bioactive hydrogels combined with dental pulp stem cells for the repair of large gap peripheral nerve injuries. Bioact Mater 2021, 6(3): 638-654.
- Martens W, Sanen K, Georgiou M, Struys T, Bronckaers A, Ameloot M, Phillips J, Lambrichts I: Human dental pulp stem cells can differentiate into Schwann cells and promote and guide neurite outgrowth in an aligned tissue-engineered collagen construct in vitro. Faseb j 2014, 28(4): 1634-1643.
- Sasaki R, Watanabe Y, Yamato M, Okamoto T: Tissue-engineered nerve guides with mesenchymal stem cells in the facial nerve regeneration. Neurochem Int 2021, 148: 105062.
- Askari N, Yaghoobi MM, Shamsara M, Esmaeili-Mahani S: Tetracycline-regulated expression of OLIG2 gene in human dental pulp stem cells lead to mouse sciatic nerve regeneration upon transplantation. Neuroscience 2015, 305: 197-208.
- Rai S, Kaur M, Kaur S, Arora SP: Redefining the potential applications of dental stem cells: An asset for future. Indian J Hum Genet 2012, 18(3): 276-284.
- Munemasa Y, Kitaoka Y: Autophagy in axonal degeneration in glaucomatous optic neuropathy. Prog Retin Eye Res 2015, 47: 1-18.
- Park M, Kim HM, Shin HA, Lee SH, Hwang DY, Lew H: Human Pluripotent Stem Cell-Derived Neural Progenitor Cells Promote Retinal Ganglion Cell Survival and Axon Recovery in an Optic Nerve Compression Animal Model. Int J Mol Sci 2021, 22(22): 12529.
- Alsaeedi HA, Koh AE, Lam C, Rashid MBA, Harun MHN, Saleh M, Teh SW, Luu CD, Ng MH, Isa HM et al: Dental pulp stem cells therapy overcome photoreceptor cell death and protects the retina in a rat model of sodium iodate-induced retinal degeneration. J Photochem Photobiol B 2019, 198: 111561.
- Tan H, Kang X, Lu S, Liu L: The therapeutic effects of bone marrow mesenchymal stem cells after optic nerve damage in the adult rat. Clin Interv Aging 2015, 10: 487-490.
- Mead B, Logan A, Berry M, Leadbeater W, Scheven BA: Paracrine-mediated neuroprotection and neuritogenesis of axotomised retinal ganglion cells by human dental pulp stem cells: comparison with human bone marrow and adipose-derived mesenchymal stem cells. PLoS One 2014, 9(10): e109305.
- Mead B, Hill LJ, Blanch RJ, Ward K, Logan A, Berry M, Leadbeater W, Scheven BA: Mesenchymal stromal cell-mediated neuroprotection and functional preservation of retinal ganglion cells in a rodent model of glaucoma. Cytotherapy 2016, 18(4): 487-496.
- Lam C, Alsaeedi HA, Koh AE, Harun MHN, Hwei ANM, Mok PL, Luu CD, Yong TK, Subbiah SK, Bastion MC: Human Dental Pulp Stem Cells (DPSCs) Therapy in Rescuing Photoreceptors and Establishing a Sodium Iodate-Induced Retinal Degeneration Rat Model. Tissue Eng Regen Med 2021, 18(1): 143-154.
- Belviso I, Di Meglio F, Romano V, Montagnani S, Castaldo C: Non-modified RNA-Based Reprogramming of Human Dermal Fibroblasts into Induced Pluripotent Stem Cells. Methods Mol Biol 2022, 2454: 675-684.
- Fehily B, Fitzgerald M: Repeated Mild Traumatic Brain Injury: Potential Mechanisms of Damage. Cell Transplant 2017, 26(7): 1131-1155.
- Jiang L, Li R, Tang H, Zhong J, Sun H, Tang W, Wang H, Zhu J: MRI Tracking of iPS Cells-Induced Neural Stem Cells in Traumatic Brain Injury Rats. Cell Transplant 2019, 28(6): 747-755.
- Nieves MD, Furmanski O, Doughty ML: Host sex and transplanted human induced pluripotent stem cell phenotype interact to influence sensorimotor recovery in a mouse model of cortical contusion injury. Brain Res 2020, 1748: 147120.
- Pilipović K, Harej Hrkać A, Kučić N, Mršić-Pelčić J: Modeling Central Nervous System Injury In Vitro: Current Status and Promising Future Strategies. Biomedicines 2022, 11(1): 94.
- Shi W, Dong P, Kuss MA, Gu L, Kievit F, Kim HJ, Duan B: Design and Evaluation of an In Vitro Mild Traumatic Brain Injury Modeling System Using 3D Printed Mini Impact Device on the 3D Cultured Human iPSC Derived Neural Progenitor Cells. Adv Healthc Mater 2021, 10(12): e2100180.
- Bhat IA, T BS, Somal A, Pandey S, Bharti MK, Panda BSK, B I, Verma M, J A, Sonwane A et al: An allogenic therapeutic strategy for canine spinal cord injury using mesenchymal stem cells. J Cell Physiol 2019, 234(3): 2705-2718.
- Anjum A, Yazid MD, Fauzi Daud M, Idris J, Ng AMH, Selvi Naicker A, Ismail OHR, Athi Kumar RK, Lokanathan Y: Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int J Mol Sci 2020, 21(20): 7533.
- Eli I, Lerner DP, Ghogawala Z: Acute Traumatic Spinal Cord Injury. Neurol Clin 2021, 39(2): 471-488.
- Lavoie NS, Truong V, Malone D, Pengo T, Patil N, Dutton JR, Parr AM: Human induced pluripotent stem cells integrate, create synapses and extend long axons after spinal cord injury. J Cell Mol Med 2022, 26(7): 1932-1942.
- Li J, Jing Y, Bai F, Wu Y, Wang L, Yan Y, Jia Y, Yu Y, Jia B, Ali F: Induced pluripotent stem cells as natural biofactories for exosomes carrying miR-199b-5p in the treatment of spinal cord injury. Front Pharmacol 2022, 13: 1078761.
- Salewski RP, Mitchell RA, Li L, Shen C, Milekovskaia M, Nagy A, Fehlings MG: Transplantation of Induced Pluripotent Stem Cell-Derived Neural Stem Cells Mediate Functional Recovery Following Thoracic Spinal Cord Injury Through Remyelination of Axons. Stem Cells Transl Med 2015, 4(7): 743-754.
- Kawabata S, Takano M, Numasawa-Kuroiwa Y, Itakura G, Kobayashi Y, Nishiyama Y, Sugai K, Nishimura S, Iwai H, Isoda M et al: Grafted Human iPS Cell-Derived Oligodendrocyte Precursor Cells Contribute to Robust Remyelination of Demyelinated Axons after Spinal Cord Injury. Stem Cell Reports 2016, 6(1): 1-8.
- Zheng Y, Gallegos CM, Xue H, Li S, Kim DH, Zhou H, Xia X, Liu Y, Cao Q: Transplantation of Human Induced Pluripotent Stem Cell-Derived Neural Progenitor Cells Promotes Forelimb Functional Recovery after Cervical Spinal Cord Injury. Cells 2022, 11(17): 2765.
- Amemori T, Ruzicka J, Romanyuk N, Jhanwar-Uniyal M, Sykova E, Jendelova P: Comparison of intraspinal and intrathecal implantation of induced pluripotent stem cell-derived neural precursors for the treatment of spinal cord injury in rats. Stem Cell Res Ther 2015, 6: 257.
- Sugai K, Fukuzawa R, Shofuda T, Fukusumi H, Kawabata S, Nishiyama Y, Higuchi Y, Kawai K, Isoda M, Kanematsu D et al: Pathological classification of human iPSC-derived neural stem/progenitor cells towards safety assessment of transplantation therapy for CNS diseases. Mol Brain 2016, 9(1): 85.
- Turón-Viñas E, Boronat S, Trabazo M, Brió S, Coca E, Morón G, Badell I: Neurologic Complications in Pediatric Allogeneic Stem Cell Transplantation: Analysis of Risk Factors and Outcome. J Child Neurol 2022, 37(2): 141-150.
- Arora D, Robey PG: Recent updates on the biological basis of heterogeneity in bone marrow stromal cells/skeletal stem cells. Biomater Transl 2022, 3(1): 3-16.
- Chen Y, An X, Wang Z, Guan S, An H, Huang Q, Zhang H, Liang L, Huang B, Wang H et al: Transcriptome and lipidome profile of human mesenchymal stem cells with reduced senescence and increased trilineage differentiation ability upon drug treatment. Aging (Albany NY) 2021, 13(7): 9991-10014.
- Zheng YL: Some Ethical Concerns About Human Induced Pluripotent Stem Cells. Sci Eng Ethics 2016, 22(5): 1277-1284.
- Burns AJ, Thapar N: Neural stem cell therapies for enteric nervous system disorders. Nat Rev Gastroenterol Hepatol 2014, 11(5): 317-328.
- Bonaventura G, Iemmolo R, Attaguile GA, La Cognata V, Pistone BS, Raudino G, D'Agata V, Cantarella G, Barcellona ML, Cavallaro S: iPSCs: A Preclinical Drug Research Tool for Neurological Disorders. Int J Mol Sci 2021, 22(9): 4596.
- Okano H, Yamanaka S: iPS cell technologies: significance and applications to CNS regeneration and disease. Mol Brain 2014, 7: 22.
- Chang KH, Lee-Chen GJ, Huang CC, Lin JL, Chen YJ, Wei PC, Lo YS, Yao CF, Kuo MW, Chen CM: Modeling Alzheimer's Disease by Induced Pluripotent Stem Cells Carrying APP D678H Mutation. Mol Neurobiol 2019, 56(6): 3972-3983.
- Aboul-Soud MAM, Alzahrani AJ, Mahmoud A: Induced Pluripotent Stem Cells (iPSCs)-Roles in Regenerative Therapies, Disease Modelling and Drug Screening. Cells 2021, 10(9): 2319.
Asia-Pacific Journal of Surgical & Experimental Pathology
ISSN 2977-5817 (Online)
 Copyright © Asia Pac J Surg Exp & Pathol. This
work is licensed under a Creative Commons AttributionNonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0)
License.
Copyright © Asia Pac J Surg Exp & Pathol. This
work is licensed under a Creative Commons AttributionNonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0)
License.

 Submit Manuscript
Submit Manuscript