Review Article | Open Access
The influence of the gut-brain axis on neurological and psychiatric well-being
Muhammad Sufyan1, Mohammad Amjad Kamal2
1Department of the Bioinformatics and Biotechnology, government College University Faisalabad, Pakistan.
2Future Technologies Research Centre, King Faisal University, AI Ahsa, Saudi Arabia.
Correspondence: Mohammad Amjad Kamal (Future Technologies Research Centre, King Faisal University, AI Ahsa, PO Box 400, Post Code 31982, Saudi Arabia; E-mail: aakamal@kfu.edu.sa).
Asia-Pacific Journal of Surgical & Experimental Pathology 2025, 2: 58-72. https://doi.org/10.32948/ajsep.2025.08.18
Received: 15 Aug 2025 | Accepted: 08 Oct 2025 | Published online: 21 Oct 2025
Key words gut-brain axis, central nervous system, neuroinflammation, personalized microbiome medicine
The gut-brain axis is best conceptualized as an extensive, bidirectional communication network. The gut-brain axis correlates the emotional and cognitive centers of the brain with the peripheral functional activities of the gastrointestinal tract [4]. This sophisticated system ensures the integration of gut homeostasis with brain function. The brain influences the gut primarily through the autonomic nervous system (ANS). It regulates primary functions like motility, secretion, and blood flow [5]. Howevr, stress-mediated activation of the hypothalamic-pituitary-adrenal (HPA) axis can smoothly alter gut permeability and microbial composition [6]. On the other hand, the gut exerts a powerful influence on the brain by sending myriad signals that can influence mood, cognition, and behavior [7]. This bottom-up signaling is largely driven by the gut's intrinsic nervous system. The enteric nervous system (ENS), often called the "second brain," which contains over 100 million neurons [7]. The longest cranial nerve is vagus nerve, serves as a primary physical conduit for this regard, transmitting visceral sensory information directly to the brainstem [8, 9]. This continuous, two-way traffic ensures that our mental state can affect gut feelings, and conversely, our gut health can significantly influence our state of mind.
The action of the gut-brain axis is mediated through several parallel and interconnected signaling pathways, ensuring robust communication [10]. These can be classified into neural, endocrine, immune, and metabolic routes. The neural pathway is primarily executed by the vagus nerve. It transmits afferent signals regarding gut state such as distension, nutrient availability, and microbial activity directly to the brainstem [11, 12]. Efferent signals from the brain then modulate gut function in response. The endocrine (or hormonal) pathway linked to gut enteroendocrine cells, and promotes secretion of serotonin (5-HT) and peptide YY (PYY) in response to nutritional and microbial cues [13]. These hormones enter circulation to influence brain function or act locally on vagal terminals. The immune pathway is critical, the gut mucosa hosts approximately 70% of the body's immune cells. Dysbiosis can enhance pro-inflammatory cytokines release (e.g., IL-1β, IL-6, TNF-α), which can pass the blood-brain barrier (BBB) or activate its endothelial cells to induce neuroinflammation [14]. Finally, metabolic pathways are associated with small molecules, particularly microbial metabolites, which serve as potent systemic messengers.
Importantly, at the core of the modern understanding of the gut-brain axis resides the gut microbiota: a vast, diverse ecosystem of trillions of bacteria, viruses, fungi, and archaea [15]. This microbial community is not a passive passenger but an active endocrine organ that fundamentally maintains axis communication. It influences brain physiology and functions via multiple mechanisms. Firstly, gut bacteria are vast biochemical factories, producing a broad array of neuroactive metabolites [16, 17]. These include short-chain fatty acids (SCFAs) like butyrate, propionate, and acetate from dietary fiber fermentation. These metabolites exert anti-inflammatory characteristics and can strengthen the blood-brain barrier [18, 19]. Secondly, microbiota plays important roles producing key neurotransmitters; specific strains can synthesize gamma-aminobutyric acid (GABA), serotonin, dopamine, and acetylcholine, which influence host neurotransmission [20, 21]. Furthermore, the microbiota is indispensable for the proper development and function of host immune system. Microbiota play important roles on host immune system, educating immune cells and preventing inappropriate inflammation that could adversely affect the brain [22]. The gut microbiota establishes itself as a central regulator. It induces the complex symphony of the gut-brain axis by modulating the production of signaling molecules, regulating immune responses, and maintaining gut barrier integrity. This review article highlights the thorough mechanisms that underpin the GBA, focusing on the pivotal role of the gut microbiota. Herein, we present current evidence on how this gut-brain communication influences brain health and disease.
A complex multi-channel network of signaling pathways facilitate the gut and the brain axis. These mechanisms ensure a constant flow of communication, allowing the brain to monitor gut activity. Also, the gut exerts a profound influence on brain function and behavior [23]. The primary routes of this communication can be classified into endocrine, neural, immune, and metabolic pathways. Importantly, the most direct line of communication is the neural pathway which is primarily mediated by the vagus nerve. This cranial nerve serves transmitting sensory information from the gut lumen (such as nutrient status and microbial activity) directly to the brainstem [24]. However, efferent signals from the brain modulate gut functions like motility and secretion. Besides, the endocrine pathway involves gut enteroendocrine cells that release neuroactive hormones, such as serotonin and peptide YY, into the bloodstream in response to nutritional and microbial cues [13, 25]. These hormones directly influence brain regions contributes in mood and appetite regulation. Thus, the most crucial pathway for pathology is the immune system [26, 27]. The gut mucosa houses a vast portion of the body's immune cells. Dysbiosis mediates intestinal integrity, leading to a "leaky gut" that allows bacterial fragments like lipopolysaccharide (LPS) to enter circulation [28, 29]. This facilitates a systemic inflammatory response, producing cytokines that can cross the blood-brain barrier or activate its cells, leading to neuroinflammation a key contributor to numerous neurological and psychiatric disorders [30, 31]. The gut microbiota produces small molecules involves in the metabolic pathway. Key small molecules are SCFAs like butyrate, which possess anti-inflammatory properties and can strengthen the blood-brain barrier, directly influencing brain health and function (Figure 1).
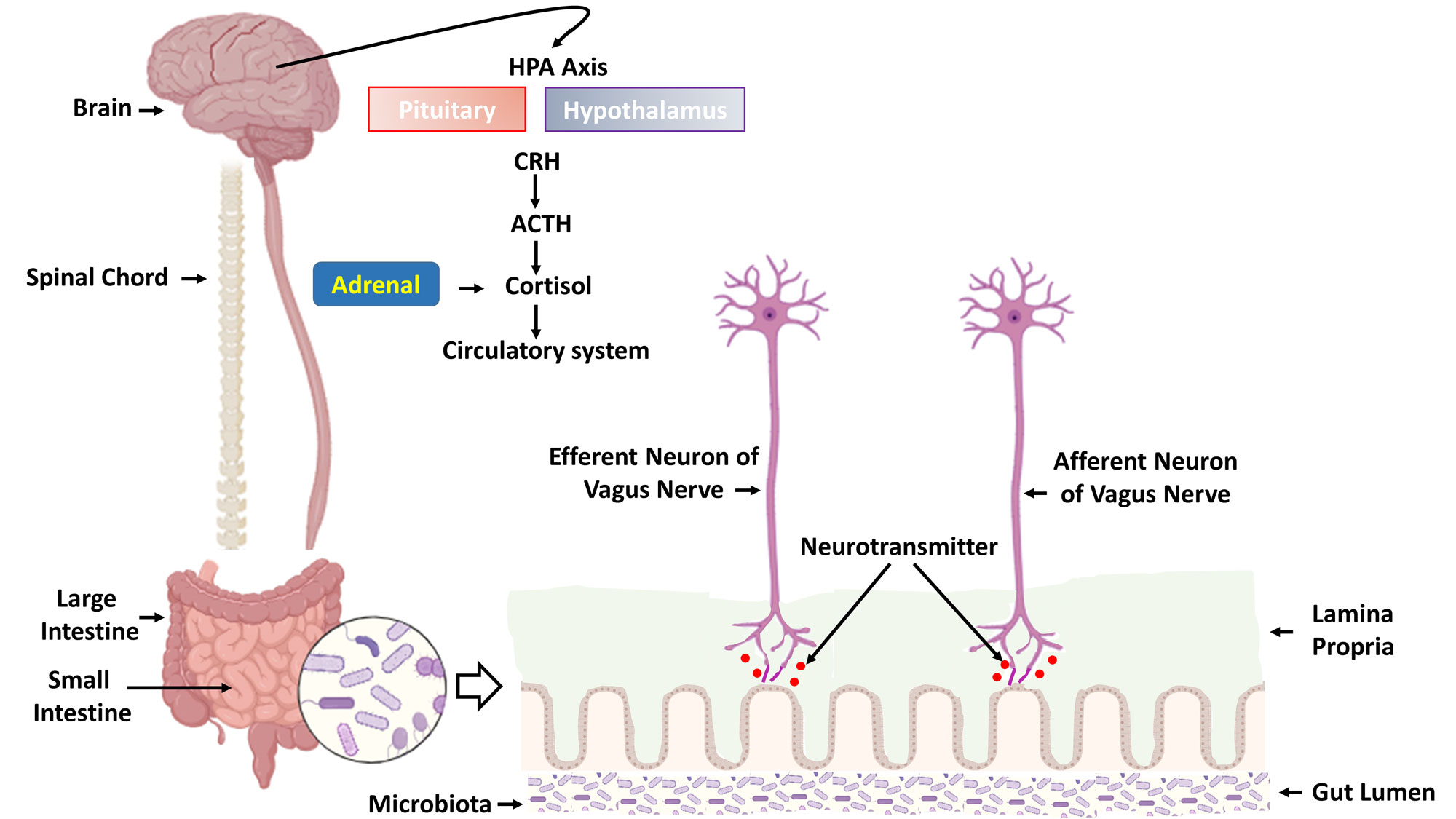 Figure 1. The integrated communication pathways of the gut-brain axis. This schematic illustrates the bidirectional signaling network connecting the Central Nervous System (CNS), comprising the Brain and Spinal Cord, with the gastrointestinal tract. The Hypothalamus, Pituitary, and Adrenal glands form the HPA axis, the body's central stress response system, which is modulated by gut-derived signals. Key communication occurs via the Vagus Nerve, where Afferent neurons relay visceral information from the gut to the CNS, and Efferent neurons carry regulatory commands back to the gut. Within the intestinal wall, the Lamina Propria houses immune cells and nerve fibers that interact with the Gut Lumen, where the Microbiota reside. These microbes produce Neurotransmitters and metabolites that influence local and central nervous system function, creating a continuous feedback loop between the Small Intestine and Large Intestine and the brain, fundamental to both neurological and psychiatric well-being.
Figure 1. The integrated communication pathways of the gut-brain axis. This schematic illustrates the bidirectional signaling network connecting the Central Nervous System (CNS), comprising the Brain and Spinal Cord, with the gastrointestinal tract. The Hypothalamus, Pituitary, and Adrenal glands form the HPA axis, the body's central stress response system, which is modulated by gut-derived signals. Key communication occurs via the Vagus Nerve, where Afferent neurons relay visceral information from the gut to the CNS, and Efferent neurons carry regulatory commands back to the gut. Within the intestinal wall, the Lamina Propria houses immune cells and nerve fibers that interact with the Gut Lumen, where the Microbiota reside. These microbes produce Neurotransmitters and metabolites that influence local and central nervous system function, creating a continuous feedback loop between the Small Intestine and Large Intestine and the brain, fundamental to both neurological and psychiatric well-being.
Accumulating evidence suggested the critical role of GBA dysfunction in the pathogenesis and progression of major neurological disorders [32]. The neural, immune, and metabolic communication channels between the gut and brain become conduits for both detrimental and protective influences. This mechanism manifests new perspectives on disease origins and potential therapeutic avenues. In Parkinson’s disease (PD), the GBA is central player to the "dual-hit" hypothesis, which proposes that an unknown pathogen may trigger the misfolding of alpha-synuclein protein first in the gut’s enteric nervous system [33, 34]. This pathology is then thought to propagate via the vagus nerve to the brainstem and ultimately the substantia nigra [35]. Additionally, individuals with vagotomies show a reduced risk of PD, and specific gut microbial profiles. It is characterized by increased pro-inflammatory species and decreased SCFA-producers, are consistently identified in patients [36, 37]. These microbial shifts promote inflammation and exacerbate alpha-synuclein aggregation.
Similarly, in Alzheimer’s disease (AD), gut dysbiosis is linked to core disease mechanisms [38]. Certain microbiota influence the production and clearance of amyloid-beta peptides through the modulation of the blood-brain barrier and systemic inflammation [39]. Moreover, microbial metabolites including pro-inflammatory molecules from detrimental bacteria and a deficiency of the anti-inflammatory short-chain fatty acid butyrate, are implicated in driving neuroinflammation and tau phosphorylation [40, 41]. The GBA also plays a role in stroke outcomes, conversely, pre- and post-stroke microbiota composition influences the severity of brain injury and the effectiveness of recovery through immune modulation [42]. Furthermore, alterations in the gut microbiome have been observed in autism spectrum disorder (ASD) and multiple sclerosis (MS), whereas microbial metabolites appear to influence neurodevelopment, immune activation, and demyelination [43, 44]. This collective evidence demonstrates the GBA not as a peripheral player, but as a fundamental component in understanding the complex etiology and progression of a wide spectrum of neurological conditions.The GBA also critically modulates the body's central stress response, the HPA axis [51]. The gut microbiota is essential for its healthy development in infancy and its regulation in adulthood [52]. Dysbiosis promotes HPA axis hyperactivity, resulting in exaggerated cortisol release and a heightened physiological response to stress, which is a well-established risk factor for anxiety and depression [53, 54]. This is specifically relevant in the context of early-life adversity. Whereas, stress-induced alterations to the microbiome can create a lifelong vulnerability to psychiatric illness. Furthermore, emerging research in ASD suggests a strong gut-brain connection. Conversely, microbial imbalances contribute to behavioral symptoms through the generation of metabolites that alter neuronal function and immune communication [55]. Therefore, this collective evidence strongly suggests the gut microbiome is an active contributor to psychiatric well-being. Besides, gut microbiome manifests promising avenues for microbiome-targeted interventions like psychobiotics and dietary strategies to augment mental health (Table 1) [56-65].
|
Table 1. Neurodegenerative & psychiatric diseases and the microbiome. |
|||
|
Disease |
Associated microbiome (Dysbiosis) |
Hypothesized role of the microbiome |
References |
|
Alzheimer's Disease (AD) |
• Reduced diversity. |
• LPS / Inflammation: Gut LPS → systemic inflammation → microglial activation → neuroinflammation. |
[56, 57] |
|
Parkinson's Disease (PD) |
• Reduced diversity. |
• Pathogen Propagation: Gut inflammation → α-syn misfolding in gut → vagus nerve propagation to brain. |
[58, 59] |
|
Autism Spectrum Disorder (ASD) |
• Reduced diversity. |
• Toxic Metabolites: Bacterial propionic acid → BBB crossing → neuroinflammation & mitochondrial dysfunction. |
[60, 61] |
|
Major Depressive Disorder (MDD) |
• Reduced diversity. |
• Cytokine Release: Pro-inflammatory microbes → IL-6, TNF-α release → HPA axis overactivation & reduced neurogenesis. |
[62, 63] |
|
Anxiety Disorders |
• Reduced diversity. |
• Vagal GABA Modulation: Specific microbes → vagus nerve signaling → altered GABA receptor expression in amygdala. |
|
|
Multiple Sclerosis (MS) |
• Reduction in SCFA-producing Clostridia. |
• Immune Cell Deficit: Low SCFAs → impaired Treg cell generation → loss of autoimmune suppression. |
[64, 65] |
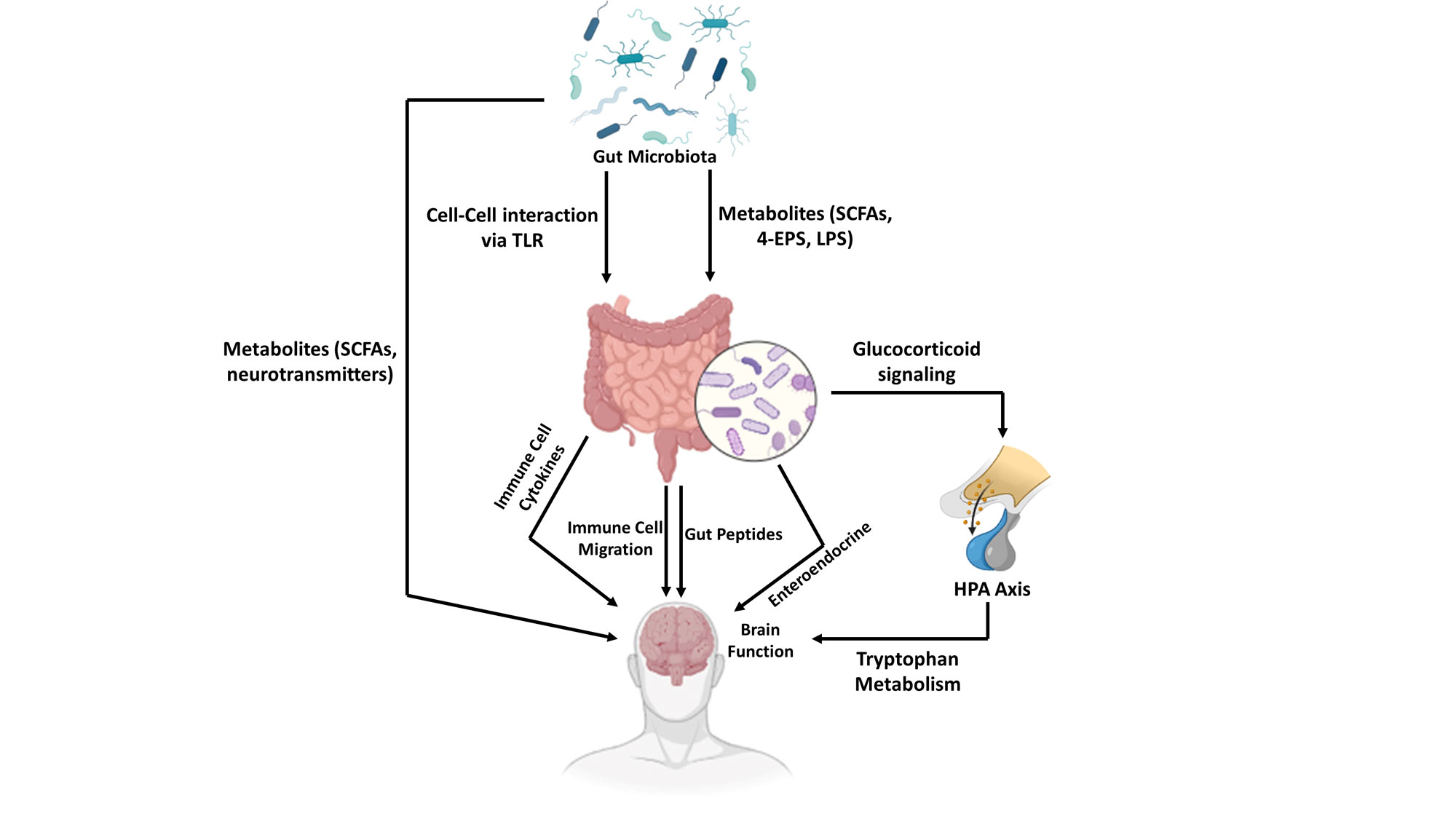 Figure 2. Molecular and Cellular Pathways of Gut-Brain Axis Communication. This schematic detail the key mechanisms by which the gut microbiota signals the brain. Commensal bacteria produce diverse Metabolites, including beneficial SCFA, neuroactive compounds (Neurotransmitter), and the pro-inflammatory LPS. These molecules mediate Cell-Cell interaction via TLR on host cells, triggering Immune cell cytokines and systemic Immune cell migration, potentially driving neuroinflammation. Enteroendocrine cells sense these signals and release Gut Peptides that act on local nerves or enter circulation. The microbiota also critically regulates Tryptophan Metabolism, influencing the production of neuroactive kynurenine pathway metabolites. Collectively, these pathways modulate Brain Function and the HPA Axis, leading to Glucocorticoid release, which provides feedback to the gut, completing a bidirectional loop central to neurological and psychiatric health.
Figure 2. Molecular and Cellular Pathways of Gut-Brain Axis Communication. This schematic detail the key mechanisms by which the gut microbiota signals the brain. Commensal bacteria produce diverse Metabolites, including beneficial SCFA, neuroactive compounds (Neurotransmitter), and the pro-inflammatory LPS. These molecules mediate Cell-Cell interaction via TLR on host cells, triggering Immune cell cytokines and systemic Immune cell migration, potentially driving neuroinflammation. Enteroendocrine cells sense these signals and release Gut Peptides that act on local nerves or enter circulation. The microbiota also critically regulates Tryptophan Metabolism, influencing the production of neuroactive kynurenine pathway metabolites. Collectively, these pathways modulate Brain Function and the HPA Axis, leading to Glucocorticoid release, which provides feedback to the gut, completing a bidirectional loop central to neurological and psychiatric health.
The ENS plays important role in neurological and psychiatric disorders. The ENS is a major site of neurotransmitter production. The ENS synthesizes approximately 90% of the body's serotonin, a key regulator of mood, appetite, and sleep [71]. Pathologically, it is implicated as a potential starting point for diseases like Parkinson's. In Parkinson's misfolded alpha-synuclein protein is believed to first aggregate in ENS neurons before propagating to the brain via the vagus nerve [72, 73]. Furthermore, ENS integrity is crucial for maintaining the gut barrier. Dysfunction of gut contributes to "leaky gut," inflammation, and the subsequent systemic immune responses leading to several neuropsychiatric conditions [49, 74]. Importantly, the ENS is a central player in translating gut microbiome activity into signals that profoundly impact the brain [75].
Furthermore, the early-life microbiome is integral to the action of the HPA axis, the body’s central stress response system [84]. Importantly, a healthy, diverse gut microbiota helps to establish appropriate stress reactivity and resilience [85, 86]. Conversely, early-life adversity, antibiotic use, or pathogenic infections can cause dysbiosis, leading to microglial dysfunction, an exaggerated HPA axis response, and altered neurodevelopment [87, 88]. The etiology of neurodevelopmental disorders such as ASD and ADHD were initiated due to the dysregulation of microbiota. It highlights that the seeds of future neurological and psychiatric health are sown within the first years of life [89, 90].
In addition, non-antibiotic drugs also exhibit significant antimicrobial actions [98]. It was found to gut microbial populations were altered dramatically due to the action of Proton-pump inhibitors (PPIs), metformin, and non-steroidal anti-inflammatory medications (NSAIDs) [99]. PPIs alter pH of the gut, which can lead to the overgrowth of orally-derived bacteria and potentially increasing infection risk [100, 101]. Importantly, many psychoactive medications, including antidepressants and antipsychotics, demonstrate antimicrobial properties in vitro [102, 103]. A compelling hypothesis suggests that a part of their potent efficacy may stem not from direct action on neurons. It indirectly through modifying the gut microbiome and its production of neurotransmitters like serotonin or GABA, thereby influencing the GBA [1, 32, 104]. Herein, we highlights a paradigm shift: the gut microbiome must be considered a primary mediator of both the therapeutic benefits and adverse effects of pharmacological interventions [105, 106]. Therefore, understanding these interactions is essential for predicting side effects, elucidating individual variability in drug response, and developing novel strategies that combine traditional therapeutics with microbiome-enhancing co-treatments to enhance patient outcomes in neurological and psychiatric care (Table 2) [107-120].
|
Table 2. Mechanisms, messengers, and effects in the gut-brain axis. |
||||
|
Component |
Key elements |
Primary function in GBA |
Effect on neurological & psychiatric health |
References |
|
Primary Communication Pathways |
Neural (Vagus Nerve): Main physical conduit for bidirectional signals. |
Transmits information between the gut and brain. Neural is direct and fast; endocrine/immune are slower, systemic; metabolic is a foundational biochemical influence. |
Ensures integration of gut homeostasis with brain function. Dysregulation in any pathway is a mechanism for disease. |
[107, 108] |
|
Key Microbial Metabolites |
Short-Chain Fatty Acids (SCFAs): Butyrate, Propionate, Acetate. |
SCFAs: Anti-inflammatory, energy for colonocytes, strengthen BBB. |
Protective (Eubiosis): SCFAs and neuroprotective kynurenic acid support brain health. |
[109, 110] |
|
Gut Barrier Integrity |
Intestinal Epithelium: Single layer of cells with tight junctions. |
Prevents translocation of bacteria and pro-inflammatory molecules (e.g., LPS) into systemic circulation. |
Intact Barrier ("Tight Gut"): Prevents systemic inflammation and neuroinflammation. |
[111, 112] |
|
Central Nervous System Barriers |
Blood-Brain Barrier (BBB): Semi-permeable lining of brain's capillaries. |
Protects the brain from toxins and pathogens in the blood while allowing nutrient passage. |
Strong BBB: Maintains a stable environment for neuronal function. |
[113, 114] |
|
Immune System Regulation |
Gut-Associated Lymphoid Tissue (GALT): Body's largest immune organ. |
The microbiome educates the immune system. Gut immune responses can activate microglia in the brain. |
Healthy Regulation: Prevents excessive inflammation; supports synaptic pruning via microglia. |
[115, 116] |
|
Stress Response System |
Hypothalamic-Pituitary-Adrenal (HPA) Axis. |
The body's central stress response system. Releases cortisol. |
Well-Regulated: The microbiome helps calibrate the HPA axis for a balanced stress response. |
[117, 118] |
|
State of the Gut Ecosystem |
Eubiosis: High diversity, balanced community, beneficial microbes dominate. |
Eubiosis supports all healthy GBA functions. |
Eubiosis: Foundation of neurological homeostasis and resilience. |
[119, 120] |
Consequently, advanced sequencing and machine learning algorithms decode microbiome fingerprint of each individual which is the future of GBA-based therapeutics [125]. The goal is to move beyond simple taxonomic analysis to a functional understanding of microbial metabolic potential of each individual. This could enable clinicians to predict disease susceptibility, tailor nutritional plans to boost specific neuroprotective metabolites, and select microbial consortia (next-generation probiotics) designed to correct a patient's specific functional deficits [126]. By acknowledging and embracing individual variability through microbiome fingerprinting, the field can progress towards developing truly effective, personalized interventions that modulate the GBA to enhance neurological resilience and psychiatric health.
Similarly, the mycobiome which is comprised of commensal fungi like Candida and Saccharomyces species, maintain balance with bacteria and the host immune system [134]. Fungi are less abundant than bacteria but potent regulators of immune responses. An overgrowth of certain fungal species can disrupt intestinal barrier integrity and amplify systemic immune activation, resulting the risk factor for neuroinflammation [14]. Furthermore, bioactive metabolites and mycotoxins are produced by some fungi that may directly influence neuronal function [135]. Focusing these interactions is crucial for a holistic understanding of how gut microbial dysbiosis impacts the brain.
Moreover, the essential amino acid tryptophan is associated with the most critical metabolic pathway. Gut microbes directly consume dietary tryptophan, but also critically regulate its host metabolism. It is very important to maintain equilibrium between the serotonin and kynurenine pathways. Microbial dysbiosis can move tryptophan away from producing serotonin, a key neurotransmitter, towards the kynurenine pathway, leading to formation of neuroactive metabolites that can be either neuroprotective (kynurenic acid) or neurotoxic (quinolinic acid) [140]. A shift towards neurotoxicity is strongly implicated in the pathogenesis of depression, anxiety, and neurodegenerative diseases. Thus, circulating metabolites provide a molecular basis for how gut microbial composition directly influences brain chemistry and vulnerability to disorder.
Moreover, the systemic influence of the gut-brain axis, demonstrating how gut microbiota dysbiosis manifests pathophysiology to peripheral organs. Driven by chronic inflammation, immune dysregulation, and aberrant microbial metabolite signaling, gut-derived alteration is mechanistically associated with a spectrum of disorders, such as, cardiovascular, renal, hepatic, pancreatic, and respiratory diseases [145]. This perspective positions the gut as a pivotal regulator of holistic organismal health (Figure 3).
Dysbiosis of human intestinal microbiota and brain homeostasis
Dysbiosis severely disrupts brain homeostasis, resulting onset of neurological and psychiatric diseases. This pathological alteration in the composition and functionality of the gut microbiota characterized by reduced biodiversity, loss of beneficial gut microbiota, and overgrowth of opportunistic pathogens [146]. As a result, signaling pathways are disrupted and this disruption potentially destabilizes microbial population, resulting impairment of brain well-being and neural function.
The potential impact of dysbiosis on brain homeostasis occurs through several interconnected mechanism. Firstly, it diminishes the intestinal epithelial barrier integrity, leading to enhancement of gut permeability. Subsequently, bacterial endotoxins, LPS enters into blood stream through gut leakage which triggers inflammation [147]. Then, onset of neuroinflammation, AD, and anxiety disorders caused by pro-inflammatory cytokines which can pass through the blood-brain barrier. Secondly, alteration of essential microbial metabolites production due to dysbiosis. Reduced production of beneficial SCFAs such as butyrate attenuates their neuroprotective, anti-inflammatory, and blood-brain barrier enhancing effects [148]. Concurrently, dysbiosis can shift tryptophan metabolism away from serotonin synthesis towards the neurotoxic kynurenine pathway, further exacerbating neurological dysfunction [149]. Furthermore, dysbiosis disrupts the regulation of the HPA axis, leading to aberrant stress responses and elevated cortisol levels that negatively influence brain regions like the hippocampus and prefrontal cortex [150]. By disruption of these vital communication networks, intestinal dysbiosis mediates synaptic plasticity, neurotransmitter balance, and glial cell function (Figure 4).
Gut microbiome on insomnia and schizophrenia
Emerging evidence reveals critical connections between gut microbiome composition and two disorders: insomnia and schizophrenia [151]. In both disorders, specific microbial alterations contribute to pathophysiology through gut-brain axis mechanisms. Subsequently, onset of immune activation, neurotransmitter production, and sleep-wake cycle regulation. Sleep pattern and quality of the sleep are altered through multiple pathways by gut microbiome diversity. Certain Lactobacillus and Bifidobacterium strains enhance GABA production, promoting relaxation and sleep initiation [152]. Conversely, microbial dysbiosis impairs normal sleep patterns and reduce sleep quality through increased formation of pro-inflammatory cytokines. In addition, the microbiome controls circadian rhythms through metabolic products that influence central clock gene expression in the hypothalamus leading alteration of sleep pattern.
In schizophrenia, microbiome alterations have been characterized by reduced microbial diversity and higher expression of pathogenic species. Alteration of microbial population is associated with onset of disease by multiple mechanisms [153]. Firstly, triggering neuroinflammation by elevated intestinal permeability allowing bacterial metabolites. Secondly, altered tryptophan metabolism and impaired dopamine and glutamate signaling. Importantly, microbial composition is affected by antipsychotic medications, that may influence therapeutic efficacy and side effects [154]. Therefore, probiotic supplementation may improve both gastrointestinal symptoms and psychological wellbeing in schizophrenia patients, possibly by reducing inflammatory markers and oxidative stress (Figure 4).
Probiotics on CNS and neurological disorders
It has been revealed that specific probiotic strains, often termed "psychobiotics", can significantly improve CNS function and potentially alleviate various neurological disorders [155]. Thus, this beneficial gut microbiota modulates neurochemical, inflammatory, and endocrine signaling. Additionally, the production of GABA, serotonin, and brain-derived neurotrophic factor (BDNF) are raised by the action probiotics which are crucial for neuronal health, synaptic plasticity, and mood regulation [156]. It was unveiled that certain Lactobacillus and Bifidobacterium strains in AD potentially reduced amyloid-beta aggregation and tau phosphorylation through anti-inflammatory mechanisms and enhanced production of neuroprotective butyrate [157]. Moreover, probiotics may improve gastrointestinal disorders by reducing systemic inflammation and alpha-synuclein aggregation in Parkinson’s disease. Specific probiotic formulations have demonstrated remarkable immunomodulatory effects in multiple sclerosis.
The meta-analyses report indicate that depression and anxiety significantly reduced by the action of particular probiotic combinations with use of anti-depressant medications [158]. Probiotics mediate effect through reduced inflammatory cytokines, normalized HPA axis activity, and elevated tryptophan availability for serotonin synthesis. Further research is needed to establish optimal strains, dosages, and treatment durations for specific neurological conditions, paving the way for more targeted microbial-based interventions in neurology and psychiatry.
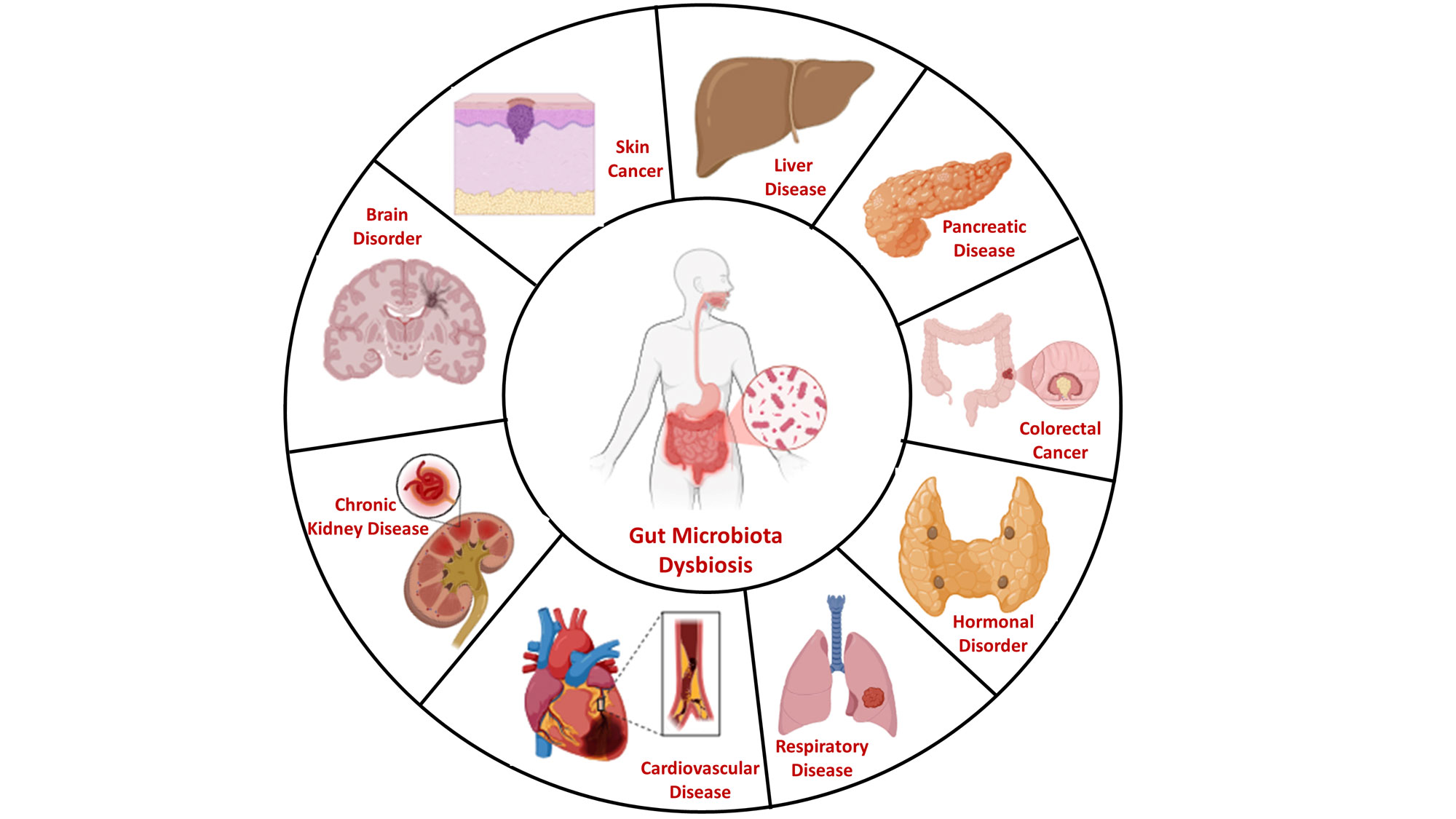 Figure 3. The Systemic reach of the gut-brain axis and connections to peripheral organ systems. This schematic illustrates how gut microbiota dysbiosis and a compromised gut-brain axis contribute to systemic pathophysiology beyond the brain. Through mechanisms of chronic systemic inflammation, immune dysregulation, and altered microbial metabolite production, gut-derived signals can disrupt the function of peripheral organs. This establishes mechanistic links between an imbalanced gut ecosystem and the development or progression of a spectrum of conditions, including cardiovascular disease, chronic kidney disease, respiratory disease, liver disease, pancreatic disease, colorectal cancer, skin cancer, hormonal disorder, and brain disorder. This holistic view underscores the gut’s role as a central modulator of whole-body health.
Figure 3. The Systemic reach of the gut-brain axis and connections to peripheral organ systems. This schematic illustrates how gut microbiota dysbiosis and a compromised gut-brain axis contribute to systemic pathophysiology beyond the brain. Through mechanisms of chronic systemic inflammation, immune dysregulation, and altered microbial metabolite production, gut-derived signals can disrupt the function of peripheral organs. This establishes mechanistic links between an imbalanced gut ecosystem and the development or progression of a spectrum of conditions, including cardiovascular disease, chronic kidney disease, respiratory disease, liver disease, pancreatic disease, colorectal cancer, skin cancer, hormonal disorder, and brain disorder. This holistic view underscores the gut’s role as a central modulator of whole-body health.
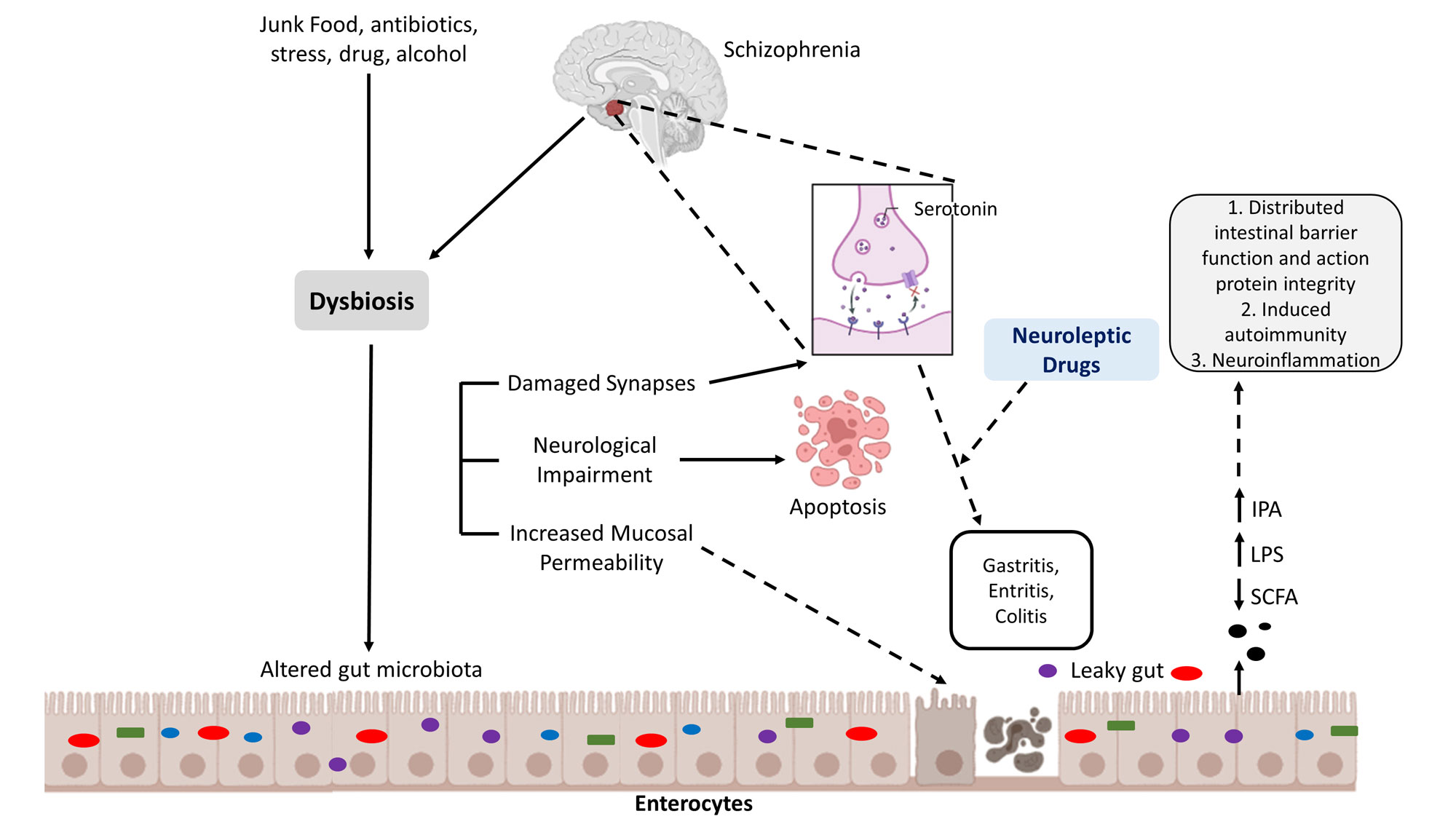 Figure 4. The vicious cycle of gut-brain dysregulation in schizophrenia pathophysiology. This schematic illustrates a proposed pathological loop linking environmental triggers, gut integrity, and brain function in Schizophrenia. Exogenous factors like junk food, stress, alcohol, antibiotics, and drug use initiate altered gut microbiota and dysbiosis. This disrupts the gut ecosystem, reducing beneficial metabolites like SCFA and IPA, while increasing harmful LPS. Consequently, increased mucosal permeability and a leaky gut cause inflammation (gastritis, enteritis, colitis) and enterocyte damage, leading to distributed intestinal barrier function and action protein integrity. Bacterial products translocate into circulation, driving systemic inflammation and neuroinflammation, which contributes to apoptosis, damaged synapses, and neurological impairment. This cycle may be further exacerbated by, or influence the efficacy of, neuroleptic drugs.
Figure 4. The vicious cycle of gut-brain dysregulation in schizophrenia pathophysiology. This schematic illustrates a proposed pathological loop linking environmental triggers, gut integrity, and brain function in Schizophrenia. Exogenous factors like junk food, stress, alcohol, antibiotics, and drug use initiate altered gut microbiota and dysbiosis. This disrupts the gut ecosystem, reducing beneficial metabolites like SCFA and IPA, while increasing harmful LPS. Consequently, increased mucosal permeability and a leaky gut cause inflammation (gastritis, enteritis, colitis) and enterocyte damage, leading to distributed intestinal barrier function and action protein integrity. Bacterial products translocate into circulation, driving systemic inflammation and neuroinflammation, which contributes to apoptosis, damaged synapses, and neurological impairment. This cycle may be further exacerbated by, or influence the efficacy of, neuroleptic drugs.
The growing interest of the GBA has increased the exploration of novel therapeutic strategies aimed to improve neurological and psychiatric well-being. These interventions target the gut microbiome and its outputs, offer a promising neurological and psychiatric treatments for the patient care. Current research focuses primarily on dietary modifications, prebiotics, and probiotics. Specific diets, particularly those high in fiber and polyphenols like the Mediterranean diet, are shown to increase microbial diversity and the production of beneficial metabolites, such as SCFAs, which confer neuroprotective and anti-inflammatory effects [159]. Targeted psychobiotics live organisms that produce health benefits in patients with psychiatric illnesses are being investigated for their potential to alleviate symptoms of depression and anxiety [160].
Importantly, future studies should be done to prioritize large-scale, longitudinal human trials to establish causal relationships between microbial changes and clinical outcomes. By integrating an individual’s unique microbial fingerprint, genetic background, and lifestyle factors, therapies can be tailored for maximum efficacy. This may involve designing specific synbiotic (combined prebiotic and probiotic) formulations or using phage therapy to precisely modulate bacterial populations. Collectively, harnessing the GBA therapeutically holds immense potential to revolutionize treatment paradigms, offering new hope for preventing and managing a spectrum of neurological and psychiatric disorders.
No applicable.
Ethics approval
No applicable.
Data availability
This narrative review is based on previously published studies and publicly available data. No new datasets were generated or analyzed for the current review.
Funding
None.
Authors’ contribution
MS contributed to the design, writing, collected data and drew figures for the manuscript. MAK revised the manuscript and approved the submission.
Competing interests
The authors declare no competing interests.
- Aljeradat B, Kumar D, Abdulmuizz S, Kundu M, Almealawy YF, Batarseh DR, Atallah O, Ennabe M, Alsarafandi M, Alan A: Neuromodulation and the gut–brain axis: therapeutic mechanisms and implications for gastrointestinal and neurological disorders. Pathophysiology 2024, 31(2): 244-268.
- Iyer G, Manoj M: NEUROBIOLOGICAL PERSPECTIVE ON THE GUT-BRAIN AXIS AND MENTAL HEALTH: A REVIEW. International Journal of Interdisciplinary Approaches in Psychology 2024, 2(8): 599: 618-599: 618.
- Gan-Or Z, Liong C, Alcalay RN: GBA-associated Parkinson’s disease and other synucleinopathies. Curr Neurol Neurosci Rep 2018, 18(8): 44.
- Mayer EA, Nance K, Chen S: The gut–brain axis. Annu Rev Med 2022, 73(1): 439-453.
- Browning KN, Travagli RA: Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr Physiol 2014, 4(4): 1339-1368.
- Doshi A: Stress modulating nutrition effect on hypothalamus pituitary adrenal axis and gut brain axis. J Nut Sci Heal Diet 2020, 1(2): 8-15.
- Mayer EA: Gut feelings: the emerging biology of gut–brain communication. Nat Rev Neurosci 2011, 12(8): 453-466.
- Ottaviani MM, Macefield VG: Structure and functions of the vagus nerve in mammals. Compr Physiol 2022, 12(4): 3989-4037.
- Prescott SL, Liberles SD: Internal senses of the vagus nerve. Neuron 2022, 110(4): 579-599.
- Aburto MR, Cryan JF: Gastrointestinal and brain barriers: unlocking gates of communication across the microbiota–gut–brain axis. Nat Rev Gastroenterol Hepatol 2024, 21(4): 222-247.
- Bonaz B, Bazin T, Pellissier S: The vagus nerve at the interface of the microbiota-gut-brain axis. Front Neurosci 2018, 12: 336468.
- Singh SC, Vayyat S, Mishra P: Gastrointestinal system and its neurophysiology. In: Brain and Organ Communication 2024: 177-188.
- Latorre R, Sternini C, De Giorgio R, Greenwood‐Van Meerveld B: Enteroendocrine cells: a review of their role in brain–gut communication. Neurogastroenterol Motil 2016, 28(5): 620-630.
- Mou Y, Du Y, Zhou L, Yue J, Hu X, Liu Y, Chen S, Lin X, Zhang G, Xiao H: Gut microbiota interact with the brain through systemic chronic inflammation: implications on neuroinflammation, neurodegeneration, and aging. Front Immunol 2022, 13: 796288.
- Heidari H, Lawrence DA: An integrative exploration of environmental stressors on the microbiome-gut-brain axis and immune mechanisms promoting neurological disorders. J Toxicol Environ Health B Crit Rev 2024, 27(7): 233-263.
- Eicher TP, Mohajeri MH: Overlapping mechanisms of action of brain-active bacteria and bacterial metabolites in the pathogenesis of common brain diseases. Nutrients 2022, 14(13): 2661.
- Wall R, Cryan JF, Ross RP, Fitzgerald GF, Dinan TG, Stanton C: Bacterial neuroactive compounds produced by psychobiotics. Adv Exp Med Biol 2014, 817: 221-39.
- Fock E, Parnova R: Mechanisms of blood–brain barrier protection by microbiota-derived short-chain fatty acids. Cells 2023, 12(4): 657.
- Silva YP, Bernardi A, Frozza RL: The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol (Lausanne) 2020, 11: 508738.
- Dicks LM: Gut bacteria and neurotransmitters. Microorganisms 2022, 10(9): 1838.
- Strandwitz P: Neurotransmitter modulation by the gut microbiota. Brain Res 2018, 1693(Pt B):128-133.
- Maranduba CMdC, De Castro SBR, Souza GTd, Rossato C, da Guia FC, Valente MAS, Rettore JVP, Maranduba CP, Souza CMd, Carmo AMRd: Intestinal microbiota as modulators of the immune system and neuroimmune system: impact on the host health and homeostasis. J Immunol Res 2015, 2015(1): 931574.
- Morais LH, Schreiber IV HL, Mazmanian SK: The gut microbiota–brain axis in behaviour and brain disorders. Nat Rev Microbiol 2021, 19(4): 241-255.
- Wang Y, Duan C, Du X, Zhu Y, Wang L, Hu J, Sun Y: Vagus nerve and gut-brain communication. The Neuroscientist 2025, 31(3): 262-278.
- Makris AP, Karianaki M, Tsamis KI, Paschou SA: The role of the gut-brain axis in depression: endocrine, neural, and immune pathways. Hormones 2021, 20(1): 1-12.
- Pizarroso NA, Fuciños P, Gonçalves C, Pastrana L, Amado IR: A review on the role of food-derived bioactive molecules and the microbiota–gut–brain axis in satiety regulation. Nutrients 2021, 13(2): 632.
- Miller GD: Appetite regulation: hormones, peptides, and neurotransmitters and their role in obesity. Am J Lifestyle Med 2019, 13(6): 586-601.
- Iacob S, Iacob DG: Infectious threats, the intestinal barrier, and its trojan horse: dysbiosis. Front Microbiol 2019, 10: 1676.
- Belizário JE, Faintuch J: Microbiome and gut dysbiosis. In: Metabolic interaction in infection. Exp Suppl 2018, 109: 459-476.
- Obrenovich ME: Leaky gut, leaky brain? Microorganisms 2018, 6(4): 107.
- Morris G, Fernandes BS, Puri BK, Walker AJ, Carvalho AF, Berk M: Leaky brain in neurological and psychiatric disorders: Drivers and consequences. Aust N Z J Psychiatry 2018, 52(10): 924-948.
- Tiwari P, Dwivedi R, Bansal M, Tripathi M, Dada R: Role of gut microbiota in neurological disorders and its therapeutic significance. J Clin Med 2023, 12(4): 1650.
- Kouli A, Torsney KM, Kuan W-L: Parkinson’s disease: etiology, neuropathology, and pathogenesis. Exon Publications 2018, Epub ahead of print.: 3-26.
- Barbut D, Stolzenberg E, Zasloff M: Gastrointestinal immunity and alpha-synuclein. J Parkinsons Dis 2019, 9(s2): S313-S322.
- Braak H, Del Tredici K: Invited Article: Nervous system pathology in sporadic Parkinson disease. Neurology 2008, 70(20): 1916-1925.
- Bolte LA, Vila AV, Imhann F, Collij V, Gacesa R, Peters V, Wijmenga C, Kurilshikov A, Campmans-Kuijpers MJ, Fu J: Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut 2021, 70(7): 1287-1298.
- Shin Y, Han S, Kwon J, Ju S, Choi TG, Kang I, Kim SS: Roles of short-chain fatty acids in inflammatory bowel disease. Nutrients 2023, 15(20): 4466.
- Liu S, Gao J, Zhu M, Liu K, Zhang H-L: Gut microbiota and dysbiosis in Alzheimer’s disease: implications for pathogenesis and treatment. Mol Neurobiol 2020, 57(12): 5026-5043.
- Deane R, Bell R, Sagare A, Zlokovic B: Clearance of amyloid-β peptide across the blood-brain barrier: implication for therapies in Alzheimer's disease. CNS & Neurological Disorders-Drug Targets-CNS & Neurological Disorders) 2009, 8(1): 16-30.
- Yao Y, Cai X, Fei W, Ye Y, Zhao M, Zheng C: The role of short-chain fatty acids in immunity, inflammation and metabolism. Crit Rev Food Sci Nutr 2022, 62(1): 1-12.
- Qian X-h, Xie R-y, Liu X-l, Chen S-d, Tang H-d: Mechanisms of short-chain fatty acids derived from gut microbiota in Alzheimer's disease. Aging Dis 2022, 13(4): 1252.
- Wang M, Liu Y, Zhong L, Wu F, Wang J: Advancements in the investigation of gut microbiota-based strategies for stroke prevention and treatment. Front Immunol 2025, 16: 1533343.
- Park J, Kim CH: Regulation of common neurological disorders by gut microbial metabolites. Exp Mol Med 2021, 53(12): 1821-1833.
- Dopkins N, Nagarkatti PS, Nagarkatti M: The role of gut microbiome and associated metabolome in the regulation of neuroinflammation in multiple sclerosis and its implications in attenuating chronic inflammation in other inflammatory and autoimmune disorders. Immunology 2018, 154(2): 178-185.
- Dezfouli MA, Rashidi SK, Yazdanfar N, Khalili H, Goudarzi M, Saadi A, Kiani Deh Kiani A: The emerging roles of neuroactive components produced by gut microbiota. Mol Biol Rep 2025, 52(1): 1.
- Di Meo F, Donato S, Di Pardo A, Maglione V, Filosa S, Crispi S: New therapeutic drugs from bioactive natural molecules: the role of gut microbiota metabolism in neurodegenerative diseases. Curr Drug Metab 2018, 19(6): 478-489.
- Dong Z, Shen X, Hao Y, Li J, Li H, Xu H, Yin L, Kuang W: Gut microbiome: a potential indicator for differential diagnosis of major depressive disorder and general anxiety disorder. Front Psychiatry 2021, 12: 651536.
- Bruun CF, Haldor Hansen T, Vinberg M, Kessing LV, Coello K: Associations between short-chain fatty acid levels and mood disorder symptoms: a systematic review. Nutr Neurosci 2024, 27(8): 899-912.
- Di Vincenzo F, Del Gaudio A, Petito V, Lopetuso LR, Scaldaferri F: Gut microbiota, intestinal permeability, and systemic inflammation: a narrative review. Intern Emerg Med 2024, 19(2): 275-293.
- da Silva Meirelles L, Simon D, Regner A: Neurotrauma: the crosstalk between neurotrophins and inflammation in the acutely injured brain. Int J Mol Sci 2017, 18(5): 1082.
- Morys J, Małecki A, Nowacka-Chmielewska M: Stress and the gut-brain axis: an inflammatory perspective. Front Mol Neurosci 2024, 17: 1415567.
- Tanaka M, Nakayama J: Development of the gut microbiota in infancy and its impact on health in later life. Allergol Int 2017, 66(4): 515-522.
- Sic A, Cvetkovic K, Manchanda E, Knezevic NN: Neurobiological implications of chronic stress and metabolic dysregulation in inflammatory bowel diseases. Diseases 2024, 12(9): 220.
- Rusch JA, Layden BT, Dugas LR: Signalling cognition: the gut microbiota and hypothalamic-pituitary-adrenal axis. Front Endocrinol (Lausanne) 2023, 14: 1130689.
- Garcia-Gutierrez E, Narbad A, Rodríguez JM: Autism spectrum disorder associated with gut microbiota at immune, metabolomic, and neuroactive level. Front Neurosci 2020, 14: 578666.
- Lin L, Zheng LJ, Zhang LJ: Neuroinflammation, gut microbiome, and Alzheimer’s disease. Mol Neurobiol 2018, 55(11): 8243-8250.
- Veerareddy V: Gut Microbial Metabolite, Sodium Butyrate Regulates the Blood-Brain Barrier Transport and Intra-Endothelial Accumulation of Alzheimer’s Disease Amyloid-Beta Peptides. University of Minnesota of Thesis of Dissertation 2024.
- Kumari S, Taliyan R, Dubey SK: Comprehensive review on potential signaling pathways involving the transfer of α-synuclein from the gut to the brain that leads to Parkinson’s disease. ACS chemical neuroscience 2023, 14(4): 590-602.
- Novakova E: The Gut-Brain Connection in Parkinson’s Disease and Its Novel Therapeutic Possibilities. Bachelor’s Degree Thesis 2023.
- Al-Ayadhi L, Zayed N, Bhat RS, Moubayed NM, Al-Muammar MN, El-Ansary A: The use of biomarkers associated with leaky gut as a diagnostic tool for early intervention in autism spectrum disorder: a systematic review. Gut Pathog 2021, 13(1): 54.
- Pulikkan J, Vasu DA, Deepthi M, Grace T: Addressing Biomedical Issues, Including Gastrointestinal Issues, Gut Dysbiosis, Immune System Dysfunction, Nutritional Deficiencies, and Metabolic Imbalance in Autism Spectrum Disorder for a Healthier and Happier Outcome [Internet]. Bridging Biology and Behavior in Autism - Innovations in Research and Practice. Intech Open; 2025.
- Zhou M, Fan Y, Xu L, Yu Z, Wang S, Xu H, Zhang J, Zhang L, Liu W, Wu L: Microbiome and tryptophan metabolomics analysis in adolescent depression: roles of the gut microbiota in the regulation of tryptophan-derived neurotransmitters and behaviors in human and mice. Microbiome 2023, 11(1): 145.
- Tian P, Chen Y, Zhu H, Wang L, Qian X, Zou R, Zhao J, Zhang H, Qian L, Wang Q: Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: A randomized clinical trial. Brain Behav Immun 2022, 100: 233-241.
- Nuss P: Anxiety disorders and GABA neurotransmission: a disturbance of modulation. Neuropsychiatr Dis Treat 2015, 11: 165-175.
- Bhargava P, Smith MD, Mische L, Harrington E, Fitzgerald KC, Martin K, Kim S, Reyes AA, Gonzalez-Cardona J, Volsko C: Bile acid metabolism is altered in multiple sclerosis and supplementation ameliorates neuroinflammation. J Clin Invest 2020, 130(7): 3467-3482.
- Niesler B, Kuerten S, Demir IE, Schäfer K-H: Disorders of the enteric nervous system—a holistic view. Nat Rev Gastroenterol Hepatol 2021, 18(6): 393-410.
- Marsilio I: Functional and Molecular Studies of the Crosstalk between Intestinal Microbioma and Enteric Nervous System and Potential Effects on the Gut-Brain Axis. University of Padua 2019, PhD Thesis.
- Furness JB: The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 2012, 9(5): 286-294.
- Yoo BB, Mazmanian SK: The enteric network: interactions between the immune and nervous systems of the gut. Immunity 2017, 46(6): 910-926.
- Giuffrè M, Moretti R, Campisciano G, da Silveira ABM, Monda VM, Comar M, Di Bella S, Antonello RM, Luzzati R, Crocè LS: You talking to me? Says the enteric nervous system (ENS) to the microbe. How intestinal microbes interact with the ENS. J Clin Med 2020, 9(11): 3705.
- Gasmi A, Nasreen A, Menzel A, Gasmi Benahmed A, Pivina L, Noor S, Peana M, Chirumbolo S, Bjørklund G: Neurotransmitters regulation and food intake: The role of dietary sources in neurotransmission. Molecules 2022, 28(1): 210.
- Casini A, Vaccaro R, Vivacqua G, Onori P, Gaudio E, Mancinelli R: Tracking the Importance of Enteric a-syn Pathology in Parkinson’s Disease. Asian J Trad Comp Alter Med 2022, 2(10): 50-57.
- Wakabayashi K: Where and how alpha‐synuclein pathology spreads in Parkinson’s disease. Neuropathology 2020, 40(5): 415-425.
- Camilleri M: Leaky gut: mechanisms, measurement and clinical implications in humans. Gut 2019, 68(8): 1516-1526.
- Heiss CN, Olofsson LE: The role of the gut microbiota in development, function and disorders of the central nervous system and the enteric nervous system. J Neuroendocrinol 2019, 31(5): e12684.
- Gershon MD, Margolis KG: The gut, its microbiome, and the brain: connections and communications. J Clin Invest 2021, 131(18): e143768.
- Knudsen EI: Sensitive periods in the development of the brain and behavior. J Cogn Neurosci 2004, 16(8): 1412-1425.
- McEwen BS, Morrison JH: The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron 2013, 79(1): 16-29.
- Gensollen T, Iyer SS, Kasper DL, Blumberg RS: How colonization by microbiota in early life shapes the immune system. Science 2016, 352(6285): 539-544.
- Kelly D, King T, Aminov R: Importance of microbial colonization of the gut in early life to the development of immunity. Mutat Res 2007, 622(1-2): 58-69.
- Tang W, Zhu H, Feng Y, Guo R, Wan D: The impact of gut microbiota disorders on the blood–brain barrier. Infect Drug Resist 2020, 29: 3351-3363.
- Hong S, Dissing-Olesen L, Stevens B: New insights on the role of microglia in synaptic pruning in health and disease. Curr Opin Neurobiol 2016, 36: 128-134.
- Bilimoria PM, Stevens B: Microglia function during brain development: new insights from animal models. Brain Research 2015, 1617: 7-17.
- Ortega VA, Mercer EM, Giesbrecht GF, Arrieta M-C: Evolutionary significance of the neuroendocrine stress axis on vertebrate immunity and the influence of the microbiome on early-life stress regulation and health outcomes. Front Microbiol 2021, 12: 634539.
- Wang J, Zhou T, Liu F, Huang Y, Xiao Z, Qian Y, Zhou W: Influence of gut microbiota on resilience and its possible mechanisms. Int J Biol Sci 2023, 19(8): 2588.
- Dogra SK, Doré J, Damak S: Gut microbiota resilience: definition, link to health and strategies for intervention. Front Microbiol 2020, 11: 572921.
- Beurel E, Nemeroff CB: Early life adversity, microbiome, and inflammatory responses. Biomolecules 2024, 14(7): 802.
- Borrego-Ruiz A, Borrego JJ: Early Life Stress and Gut Microbiome Dysbiosis: A Narrative Review. Stresses 2025, 5(2): 38.
- Scattolin MAdA, Resegue RM, Rosário MCd: The impact of the environment on neurodevelopmental disorders in early childhood. J Pediatr (Rio J) 2022, 98: 66-72.
- Bertollo AG, Puntel CF, da Silva BV, Martins M, Bagatini MD, Ignácio ZM: Neurobiological Relationships Between Neurodevelopmental Disorders and Mood Disorders. Brain Sci 2025, 15(3): 307.
- Riggott C, Ford AC, Gracie DJ: The role of the gut–brain axis in inflammatory bowel disease and its therapeutic implications. Aliment Pharmacol Ther 2024, 60(9): 1200-1214.
- Mafe AN, Büsselberg D: Microbiome integrity enhances the efficacy and safety of anticancer drug. Biomedicines 2025, 13(2): 422.
- Kearns R: Gut–brain axis and neuroinflammation: the role of gut permeability and the kynurenine pathway in neurological disorders. Cell Mol Neurobiol 2024, 44(1): 64.
- Kohanski MA, Dwyer DJ, Collins JJ: How antibiotics kill bacteria: from targets to networks. Nat Rev Microbiol 2010, 8(6): 423-435.
- Vincent C, Manges AR: Antimicrobial use, human gut microbiota and Clostridium difficile colonization and infection. Antibiotics 2015, 4(3): 230-253.
- Anand N, Gorantla VR, Chidambaram SB: The role of gut dysbiosis in the pathophysiology of neuropsychiatric disorders. Cells 2022, 12(1): 54.
- Maiuolo J, Gliozzi M, Musolino V, Carresi C, Scarano F, Nucera S, Scicchitano M, Oppedisano F, Bosco F, Ruga S: The contribution of gut microbiota–brain axis in the development of brain disorders. Front Neurosci 2021, 15: 616883.
- Lagadinou M, Onisor MO, Rigas A, Musetescu D-V, Gkentzi D, Assimakopoulos SF, Panos G, Marangos M: Antimicrobial properties on non-antibiotic drugs in the era of increased bacterial resistance. Antibiotics 2020, 9(3): 107.
- Le Bastard Q, Berthelot L, Soulillou J-P, Montassier E: Impact of non-antibiotic drugs on the human intestinal microbiome. Expert Rev Mol Diagn 2021, 21(9): 911-924.
- Damdoum M: Potential Effects of Proton Pump Inhibitors on Periodontal Disease: A Systematic Review. State University of New York at Buffalo 2028, Master of Science.
- Zhang X, Li Q, Xia S, He Y, Liu Y, Yang J, Xiao X: Proton Pump Inhibitors and Oral–Gut Microbiota: From Mechanism to Clinical Significance. Biomedicines 2024, 12(10): 2271.
- Caldara M, Marmiroli N: Antimicrobial properties of antidepressants and antipsychotics—possibilities and implications. Pharmaceuticals 2021, 14(9): 915.
- Kalaycı S, Demirci S, Sahin F: Antimicrobial properties of various psychotropic drugs against broad range microorganisms. Curr Psychopharmacol 2014, 3(3): 195-202.
- Rizzolatti G, Cattaneo L, Fabbri-Destro M, Rozzi S: Cortical mechanisms underlying the organization of goal-directed actions and mirror neuron-based action understanding. Physiol Rev 2014, 94(2): 655-706.
- Khan I, Yasir M, Azhar EI, Kumosani T, Barbour EK, Bibi F, Kamal MA: Implication of gut microbiota in human health. CNS Neurol Disord Drug Targets 2014, 13(8): 1325-1333.
- Ugwu OP-C, Alum EU, Okon MB, Obeagu EI: Mechanisms of microbiota modulation: Implications for health, disease, and therapeutic interventions. Medicine 2024, 103(19): e38088.
- Kasarello K, Cudnoch-Jedrzejewska A, Czarzasta K: Communication of gut microbiota and brain via immune and neuroendocrine signaling. Front Microbiol 2023, 14: 1118529.
- Younis NK, Alfarttoosi KH, Sanghvi G, Roopashree R, Kashyap A, Krithiga T, Taher WM, Alwan M, Jawad MJ, Al-Nuaimi AMA: The Role of Gut Microbiota in Modulating Immune Signaling Pathways in Autoimmune Diseases. Neuromolecular Med 2025, 27(1): 65.
- Zhong J-G, Lan W-T, Feng Y-Q, Li Y-H, Shen Y-Y, Gong J-H, Zou Z, Hou X: Associations between dysbiosis gut microbiota and changes of neurotransmitters and short-chain fatty acids in valproic acid model rats. Front Physiol 2023, 14: 1077821.
- Wu M, Tian T, Mao Q, Zou T, Zhou C-j, Xie J, Chen J-j: Associations between disordered gut microbiota and changes of neurotransmitters and short-chain fatty acids in depressed mice. Transl Psychiatry 2020, 10(1): 350.
- Schoultz I, Keita ÅV: The intestinal barrier and current techniques for the assessment of gut permeability. Cells 2020, 9(8): 1909.
- Takiishi T, Fenero CIM, Câmara NOS: Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue barriers 2017, 5(4): e1373208.
- Chauca JMN, Martínez GGR: Histological and Functional Breakdown of the Blood-Brain Barrier in Alzheimer’s Disease: A Multifactorial Intersection. Neurol Int 2025, 17(10): 166
- Sheikh MH, Errede M, d'Amati A, Khan NQ, Fanti S, Loiola RA, McArthur S, Purvis GSD, O'Riordan CE, Ferorelli D, et al: Impact of metabolic disorders on the structural, functional, and immunological integrity of the blood-brain barrier: Therapeutic avenues. FASEB J 2022, 36(1): e22107.
- Franco-Robles E, Ramírez-Emiliano J, López-Briones JS, Balcón-Pacheco CD: Prebiotics and the Modulation on the Microbiota-GALT-Brain Axis. In: Prebiotics and Probiotics-Potential Benefits in Nutrition and Health. IntechOpen 2019.
- Kim D-KC: Gut microbiota-immune system interactions in health and neurodegenerative diseases: Insights into molecular mechanisms and therapeutic applications. Aging Dis 2025, 16(6): 3421-3452.
- Goehler LE: Mind-Body Interactions and the Stress-Inflammation Connection. A 6-Hour Seminar for Health Professionals 2017.
- Patil A, Singh N: Stress as a Catalyst: Understanding its Role in Bowel Cancer. Preprints 2024, 2024031511.
- Bagheri M, Zahmatkesh A, Hussain A, Taj T, Ashique S, Mojgani N: Gut Microbiota: Role in Health and Disease. In: Role of Gut Microbiota and Postbiotics for Colorectal Cancer: Advancing Therapeutic Strategies. edn. Springer 2025. p1-14.
- Abraham CA: Medication provoked dysbiosis: emerging therapies targeting the micobiome. Master Degree 2025.
- Ayeni KI, Berry D, Wisgrill L, Warth B, Ezekiel CN: Early-life chemical exposome and gut microbiome development: African research perspectives within a global environmental health context. Trends Microbiol 2022, 30(11): 1084-1100.
- Arora R, Baldi A: Revolutionizing neurological disorder treatment: integrating innovations in pharmaceutical interventions and advanced therapeutic technologies. Curr Pharm Des 2024, 30(19): 1459-1471.
- Pirbaglou M, Katz J, de Souza RJ, Stearns JC, Motamed M, Ritvo P: Probiotic supplementation can positively affect anxiety and depressive symptoms: a systematic review of randomized controlled trials. Nutr Res 2016, 36(9): 889-898.
- Yang B, Wei J, Ju P, Chen J: Effects of regulating intestinal microbiota on anxiety symptoms: a systematic review. Gen Psychiatr 2019, 32(2): e100056.
- Lotter S, Mohr E, Rutsch A, Brand L, Ronchi F, Díaz-Marugán L: Synthetic MC via Biological Transmitters: Therapeutic Modulation of the Gut-Brain Axis. arXiv preprint arXiv: 250707604 2025.
- Das S, Mahajan AA, Dey A, Bhattacharya A, Bhunia S, Dasgupta R, Majumdar M, Indu N, Hait A, Ghosh S: Probiotics: Unleashing the Regenerative Potential for Neuronal Health in Neurodegenerative Diseases. In: Probiotics edn. CRC Press 2024. p227-250.
- Zaman TB: The human gut virome at the crossroads of immunity, disease and therapeutics: a comprehensive review. Brac University 2025, Bachelors Thesis.
- Chaudhary A: Role of gut virome and mycobiome in neurological disorders. In: Microbiota-Gut-Brain Axis and CNS Disorders. edn. Elsevier 2025. p383-408.
- Ogilvie LA, Jones BV: The human gut virome: a multifaceted majority. Front Microbiol 2015, 6: 918.
- Columpsi P, Sacchi P, Zuccaro V, Cima S, Sarda C, Mariani M, Gori A, Bruno R: Beyond the gut bacterial microbiota: The gut virome. J Med Virol 2016, 88(9): 1467-1472.
- Marantos A: Population Dynamics of Phage-Bacteria Ecosystems under Challenging Conditions. School of The Faculty of Science, University of Copenhagen København, Denmark 2023, PhD Thesis.
- Rasmussen TS, Koefoed AK, Jakobsen RR, Deng L, Castro-Mejía JL, Brunse A, Neve H, Vogensen FK, Nielsen DS: Bacteriophage-mediated manipulation of the gut microbiome–promises and presents limitations. FEMS Microbiol Rev 2020, 44(4): 507-521.
- Mazhar SF: The Effects of Gut Dysbiosis via Bacteriophages and its Role in Parkinson's Disease. Carleton University 2023, Master Degree Thesis.
- Underhill DM, Iliev ID: The mycobiota: interactions between commensal fungi and the host immune system. Nat Rev Immunol 2014, 14(6): 405-416.
- Zhou H, Sun F, Lin H, Fan Y, Wang C, Yu D, Liu N, Wu A: Food bioactive compounds with prevention functionalities against fungi and mycotoxins: developments and challenges. Curr Opin Food Sci 2022, 48: 100916.
- Lee B, Lee SM, Song JW, Choi JW: Gut Microbiota Metabolite Messengers in Brain Function and Pathology at a View of Cell Type-Based Receptor and Enzyme Reaction. Biomol Ther (Seoul) 2024, 32(4): 403-423.
- O'Riordan KJ, Collins MK, Moloney GM, Knox EG, Aburto MR, Fülling C, Morley SJ, Clarke G, Schellekens H, Cryan JF: Short chain fatty acids: Microbial metabolites for gut-brain axis signalling. Mol Cell Endocrinol 2022, 546: 111572.
- Bourassa MW, Alim I, Bultman SJ, Ratan RR: Butyrate, neuroepigenetics and the gut microbiome: Can a high fiber diet improve brain health? Neurosci Lett 2016, 625: 56-63.
- Wu Y, Qiu Y, Su M, Wang L, Gong Q, Wei X: Activation of the bile acid receptors TGR5 and FXR in the spinal dorsal horn alleviates neuropathic pain. CNS Neurosci Ther 2023, 29(7): 1981-1998.
- Bosi A, Banfi D, Bistoletti M, Giaroni C, Baj A: Tryptophan Metabolites Along the Microbiota-Gut-Brain Axis: An Interkingdom Communication System Influencing the Gut in Health and Disease. Int J Tryptophan Res 2020, 13: 1178646920928984.
- Afzaal M, Saeed F, Shah YA, Hussain M, Rabail R, Socol CT, Hassoun A, Pateiro M, Lorenzo JM, Rusu AV et al: Human gut microbiota in health and disease: Unveiling the relationship. Front Microbiol 2022, 13: 999001.
- Clemente-Suárez VJ, Redondo-Flórez L, Rubio-Zarapuz A, Martín-Rodríguez A, Tornero-Aguilera JF: Microbiota Implications in Endocrine-Related Diseases: From Development to Novel Therapeutic Approaches. Biomedicines 2024, 12(1): 221.
- Gwak MG, Chang SY: Gut-Brain Connection: Microbiome, Gut Barrier, and Environmental Sensors. Immune Netw 2021, 21(3): e20.
- Rusch JA, Layden BT, Dugas LR: Signalling cognition: the gut microbiota and hypothalamic-pituitary-adrenal axis. Front Endocrinol (Lausanne) 2023, 14: 1130689.
- Shen Y, Fan N, Ma SX, Cheng X, Yang X, Wang G: Gut Microbiota Dysbiosis: Pathogenesis, Diseases, Prevention, and Therapy. MedComm (2020) 2025, 6(5): e70168.
- Kandpal M, Indari O, Baral B, Jakhmola S, Tiwari D, Bhandari V, Pandey RK, Bala K, Sonawane A, Jha HC: Dysbiosis of Gut Microbiota from the Perspective of the Gut-Brain Axis: Role in the Provocation of Neurological Disorders. Metabolites 2022, 12(11): 1064.
- Chidambaram SB, Rathipriya AG, Mahalakshmi AM, Sharma S, Hediyal TA, Ray B, Sunanda T, Rungratanawanich W, Kashyap RS, Qoronfleh MW et al: The Influence of Gut Dysbiosis in the Pathogenesis and Management of Ischemic Stroke. Cells 2022, 11(7): 1239.
- Silva YP, Bernardi A, Frozza RL: The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front Endocrinol (Lausanne) 2020, 11: 25.
- Gao K, Mu CL, Farzi A, Zhu WY: Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv Nutr 2020, 11(3): 709-723.
- Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, Wesselingh S: From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatry 2016, 21(6): 738-748.
- Li Y, Deng Q, Liu Z: The relationship between gut microbiota and insomnia: a bi-directional two-sample Mendelian randomization research. Front Cell Infect Microbiol 2023, 13: 1296417.
- Li Y, Shao L, Mou Y, Zhang Y, Ping Y: Sleep, circadian rhythm and gut microbiota: alterations in Alzheimer’s disease and their potential links in the pathogenesis. Gut Microbes 2021, 13(1): 1957407.
- Tsamakis K, Galinaki S, Alevyzakis E, Hortis I, Tsiptsios D, Kollintza E, Kympouropoulos S, Triantafyllou K, Smyrnis N, Rizos E: Gut Microbiome: A Brief Review on Its Role in Schizophrenia and First Episode of Psychosis. Microorganisms 2022, 10(6): 1121.
- Nita I-B, Văcărean-Trandafir I-C, Amărandi R-M, Ilie O-D, Dobrin P-R, Bejenariu A-C, Ivanov I-C, Doroftei B: Exploring gut microbiota profile induced by antipsychotics in schizophrenic patients: insights from an Eastern European pilot study. BMC Psychiatry 2025, 25(1): 1037.
- Oroojzadeh P, Bostanabad SY, Lotfi H: Psychobiotics: the Influence of Gut Microbiota on the Gut-Brain Axis in Neurological Disorders. J Mol Neurosci 2022, 72(9): 1952-1964.
- Bathina S, Das UN: Brain-derived neurotrophic factor and its clinical implications. Arch Med Sci 2015, 11(6): 1164-1178.
- Harrass S, Quansah M, Kumar S, Radzieta M, Jayawardena B, Jones C, David M, Heng B, Elbourne LDH, Amanquah S et al: Lactobacilli Probiotics Prevent Amyloid-Beta Fibril Formation In Vitro. Probiotics Antimicrob Proteins 2025.
- Merkouris E, Mavroudi T, Miliotas D, Tsiptsios D, Serdari A, Christidi F, Doskas TK, Mueller C, Tsamakis K: Probiotics' Effects in the Treatment of Anxiety and Depression: A Comprehensive Review of 2014-2023 Clinical Trials. Microorganisms 2024, 12(2): 411.
- Nagpal R, Shively CA, Register TC, Craft S, Yadav H: Gut microbiome-Mediterranean diet interactions in improving host health. F1000Res 2019, 8: 699.
- Kamal N, Saharan BS, Duhan JS, Kumar A, Chaudhary P, Goyal C, Kumar M, Goyat N, Sindhu M, Mudgil P: Exploring the promise of psychobiotics: Bridging gut microbiota and mental health for a flourishing society. Medicine in Microecology 2025, 23: 100118.
Asia-Pacific Journal of Surgical & Experimental Pathology
ISSN 2977-5817 (Online)
 Copyright © Asia Pac J Surg Exp & Pathol. This
work is licensed under a Creative Commons AttributionNonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0)
License.
Copyright © Asia Pac J Surg Exp & Pathol. This
work is licensed under a Creative Commons AttributionNonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0)
License.

 Submit Manuscript
Submit Manuscript