Review Article | Open Access
Relationship between leptin receptor and TNF-α in obesity and polycystic ovary syndrome
Omnia Mohamed Attia1, Ahmed Attia Ahmed Abdelmoaty2
1Department of Medicine, Faculty of Medicine, Aswan University, Aswan 81528, Egypt.
2Department of Pharmacology, Faculty of Pharmacy, Zagazig University, Zagazig 44519, Egypt.
Correspondence: Ahmed Attia Ahmed Abdelmoaty (Department of Pharmacology, Faculty of Pharmacy, Zagazig University, Zagazig 44519, Egypt; E-mail: abdelmoaty14@lzu.edu.cn).
Asia-Pacific Journal of Surgical & Experimental Pathology 2025, 2: 48-57. https://doi.org/10.32948/ajsep.2025.06.20
Received: 22 Apr 2025 | Accepted: 08 Jun 2025 | Published online: 25 Aug 2025
Key words leptin receptor, TNF-α, polycystic ovarian syndrome, ovary syndrome
Obesity is not only a common complication of PCOS but also closely related to the severity of clinical manifestations of PCOS. Especially abdominal obesity which is closely related to insulin resistance and metabolic disorders in PCOS patients [5]. Studies have indicated that between 50 and 80 percent of PCOS individuals are obese, which especially if their abdominal fat is concentrated. Abnormal lipid metabolism, unbalanced blood glucose regulation and the aggravation of insulin resistance are intimately linked to this kind of abdominal obesity. escalating metabolic illnesses and insulin resistance [6]. Furthermore, research indicates that obesity and chronic low-grade inflammation are closely associated with the former contributing significantly to the onset and progression of PCOS.
TNF-α and leptin are two important variables intimately linked to inflammation and obesity [7]. Leptin is a polypeptide hormone secreted by adipocytes that regulates hunger and energy metabolism. It attaching to the leptin receptor (LEPR) in the hypothalamus, that largely lowers appetite and increases energy expenditure [8]. Even though even when blood levels of leptin rise, obese patients frequently develop leptin resistance. There is an imbalance in energy metabolism as a result of the receptors' decreased sensitivity to its effects [9]. TNF-α is a significant pro-inflammatory cytokine that is expressed in many different organs and mostly released by macrophages. It has a broad range of functions. Its expression level is strongly correlated with chronic low-grade inflammation, insulin resistance, metabolic diseases and it is markedly higher in obese and PCOS individuals [10]. Studies have indicated that TNF-α, via its receptor TNFR1, activates downstream signaling pathways. Finally unleashing NF-κB and turning on a number of immune response-related genes [11]. Increased insulin resistance is closely linked to elevated TNF-α in PCOS patients, which could exacerbate the vicious cycle of obesity and insulin resistance by interfering with insulin signaling pathway [12]. High levels of TNF-α expression are thought to be one of the indicators of persistent low-grade inflammation, which increases the risk of metabolic syndrome, particularly in obese PCOS women. Furthermore, PCOS individuals had noticeably higher levels of TNF-α and other pro-inflammatory markers (IL-6, IL-1β). It suggests that there are notable immunological and inflammatory anomalies in the PCOS etiology [13]. The abnormal expression of these inflammatory factors in ovarian tissue may lead to follicular dysplasia, ovulation disorders and reproductive dysfunction.
Due to the important roles of leptin receptor and TNF-α in obesity and PCOS. This review will explore in detail their interactions and potential pathological mechanisms in obesity related PCOS. Understanding the interaction between leptin receptors and TNF-α can help to better understand the metabolism and inflammatory pathological processes of PCOS and provide new research directions for future treatment strategies.
In 1994, Friedman et al. made the initial discovery that leptin is secreted by white adipocytes in adipose tissue. The Greek term "leptos," which means "thin," is where the word "leptin" originates. In the central nervous system, leptin functions as a peptide hormone to control hunger and energy balance [8]. Leptin secretion is directly regulated by the level of fat stores in the body. The higher the fat stores the more leptin is secreted. As a consequence, leptin becomes a “sensor” of the body’s energy stores, controlling hunger, body weight and metabolic processes through binding to certain receptors in the central nervous system, particularly in the hypothalamus [14].
Leptin suppresses appetite by activating neurons in the hypothalamic arcuate nucleus (ARC). Pro-appetite AgRP/NPY (neuropeptide Y) neurons and anti-appetite POMC (pro-opiomelanocortin) neurons make up the two primary neuronal subgroups in the arcuate nucleus [15]. The main way that leptin works is by attaching itself to LEPR receptors which then activates POMC neurons while suppressing AgRP/NPY neurons. This signaling cascade induces hypophagia and hypermetabolism [16]. Furthermore, leptin stimulates energy expenditure and lipolysis from regulating sympathetic nervous system activity.
In addition to its key role in energy metabolism, leptin plays a large number of roles in the reproductive, immune and endocrine systems. Leptin has particularly noticeable impacts on the reproductive system, it regulates reproductive function through the hypothalamic-pituitary-gonadal axis [17]. Research has indicated a clear correlation between decreased fertility and low leptin levels. For instance, Ballauff et al. demonstrated that women with anorexia nervosa had much lower levels of leptin and this was connected with significantly lower levels of reproductive hormones like luteinizing hormone (LH) and follicle stimulating hormone (FSH). Low leptin levels lead to decreased secretion of these hormones which in turn affects ovarian function and may lead to menopause and decreased fertility [18]. Conversely, in patients with PCOS, the reproductive function remains impaired due to leptin resistance. Despite the fact that leptin levels are usually high. In an experimental model, Jamal et al. discovered that low body weight in pigs overexpressing leptin negatively impacts reproductive function, resulting in uneven oestrus cycles, delayed puberty and decreased reproductive efficiency. This could be connected to immune system activation, metabolic abnormalities and changed ovarian function [19]. One of the main contributing elements to the pathomechanism of PCOS is hypothesized to be anomalies in the leptin signaling pathway.
leptin receptor
Leptin carries out its biological functions through its receptor, the leptin receptor (LEPR) which belongs to a family of type I cytokine receptors with similar structural features to other erythropoietin receptors, insulin receptors, etc [20]. There are several splice variants of LEPR in humans and it has been found that the most functionally intact and capable of leptin signaling is mainly the long chain variant (LEPRb) [21]. LEPRb is predominantly expressed in several nuclei of the hypothalamus, especially the arcuate nucleus (ARC) which is intimately associated with the regulation of energy metabolism.
The primary mechanism via which LEPRb signaling is executed is the JAK-STAT pathway. The intracellular region of LEPRb is phosphorylated by leptin after it binds to the receptor and activates the receptor-associated JAK2 kinase. Phosphorylation and activation of STAT3 are the outcomes of this mechanism. With its ability to control the transcription of the target genes linked to energy expenditure and appetite suppression, which activated STAT3 is transported to the nucleus as a transcription factor [22]. Wang et al. found that energy restriction (ER) reduced fat mass and obesity-associated (FTO) expression in the hypothalamus and brainstem, but but did not significantly modulate the expression of in peripheral tissues. The leptin receptor long isoform (LepRb) and FTO co-localize in the nuclei of the hypothalamic arcuate nucleus and isolated tractus solitarius. The hypothalamic bundle nucleus. LepRb mutant db/db mice showed defective leptin-activated STAT3 signaling pathway and total elimination of the ER-induced downregulation of FTO and weight reduction effects. The data imply that the down-regulation of FTO in the brain during ER is caused by the LepRb-STAT3 signaling pathway [23]. This study provides the first evidence of this signaling pathway in FTO regulation. In addition, LEPRb can affect glucose metabolism and insulin sensitivity through the PI3K/Akt signaling pathway [24]. Activation of the PI3K/Akt pathway plays a particularly critical role in the metabolic effects of leptin in peripheral tissues. Especially in the regulation of lipolysis, lipid metabolism and insulin signaling. It has been shown that leptin enhances insulin action through this pathway, improving glucose uptake and utilization which is important for obesity-associated insulin resistance [25, 26].
In obesity-associated PCOS, abnormalities in LEPRb signaling may result in diminished leptin regulation of the hypothalamus which in turn fails to effectively regulate appetite and energy metabolism. Abnormalities in this receptor function are particularly common in patients with PCOS, further exacerbating the worsening of insulin resistance and metabolic disorders.
Although leptin is plays a key hormone in modulating energy balanc and metabolic homeostasis, leptin resistance is often seen in obese patients. Leptin resistance refers to the fact that despite elevated blood levels of leptin its function in regulating appetite and metabolism is inhibited, which resulting in a high appetite and a failure to significantly increase energy expenditure further exacerbating weight gain.
The following are possible mechanisms of leptin resistance:
(1) Leptin transportation disorder: Leptin must enter the hypothalamus through the blood-brain barrier (BBB) in order to play a role. It has been found that the transportation efficiency of leptin through the blood-brain barrier is significantly reduced in obese patients which may be due to changes in the structure of the BBB or impaired function of transport proteins [27]. However, research has found that this barrier is expected to be broken through. The findings of this work provide insights into the regulation of hormone transport across the BBB. Recently, Shi, Y., et al. reported that 17β-estradiol was found to be a compound that significantly increases leptin transport in an iPSC-derived BBB model. Additionally, knockdown of CAT-1 expression by CRISPR-mediated epigenome editing led to a significant increase in leptin transport [28].
(2) Dysregulation of the leptin receptor signaling pathway: even if leptin is able to bind to the receptor and the obesity-associated chronic low-grade inflammatory state may interfere with LEPRb signaling pathways. especially the downstream effects of the JAK-STAT and PI3K/Akt pathways [29]. Leptin's interaction with other hormones modifies the signaling described above and triggers the release of several cytokines, which modifies the inflammatory and impacts metabolic processes. According to Barrios et al.'s data, lower NEFA levels and the activation of insulin signaling targets are linked to lower muscle inflammatory measures. This suggests that leptin may integrate these pathways [30]. Furthermore, Lee et al. showed that leptin synthesis and secretion are regulated by a variety of factors, which including insulin, steroid hormones, cytokines, norepinephrine and glucocorticoids [31]. Impairment of these signaling leads to a reduction in leptin's ability to regulate appetite, energy metabolism and glucose metabolism.
(3) Overexpression of SOCS3 protein: A leptin signaling negative feedback regulator is Suppressor of Cytokine Signaling Protein 3 (SOCS3). In the obese state, the expression level of SOCS3 was significantly elevated. It inhibiting the JAK-STAT signaling pathway and further impairing the effectiveness of leptin signaling [32]. Reed et al. demonstrated that obesity and leptin resistance can be caused solely by Socs3 overexpression in POMC neurons. Prior to the beginning of obesity, socs3 overexpression disrupts mTOR and STAT3 signaling. The absence of obesity in mice with leptin receptor neuron-induced Socs3 overexpression implies that Socs3's impact on energy balance may be cell type-specific [33]. All things considered, our findings indicate that POMC neurons play a significant role as mediators of Socs3's effects on obesity and leptin resistance.
Leptin resistance is a common pathological mechanism in obesity and PCOS. In obesity, the body's adipocytes secrete large amounts of leptin but the body's sensitivity to leptin is reduced due to factors such as chronic low-grade inflammation and insulin resistance, which resulted in the inability of leptin to perform its normal functions of suppressing appetite and promoting energy expenditure. In PCOS patients, leptin resistance is particularly pronounced further exacerbating metabolic disturbances. Leptin resistance not only increases the patient's body fat content but is also closely related to insulin resistance. So, both of which work together to cause further deterioration of glucose-fat metabolism.
PCOS can activate the immune system and have varying degrees of impact on it (Figure 1). Many types of cells secrete TNF-α, a proinflammatory cytokine, including macrophages, monocytes, T cells and adipocytes. TNF-α was originally named in 1975 for its necrotic effects on tumor cells but its broader physiological functions are reflected in the modulation of immune and inflammatory responses [34]. With binding to TNF receptors (TNF-R1 and TNF-R2), TNF-α stimulates a number of signaling pathways, such as the nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) pathways, which control cell proliferation, differentiation, apoptosis and inflammatory responses [35].
In acute inflammatory responses, TNF-α rapidly induces the release of a variety of inflammatory mediators. Such as interleukins (IL-1, IL-6) and chemokines which further enhance immune cell recruitment and activation, and are critical for pathogen clearance. The inflammatory marker C-reactive protein (CRP) interferes with leptin bioavailability and insulin sensitivity, which in turn affects energy and glucose metabolism. The metabolic profile of PCOS includes hyperleptinemia, hyperinsulinemia, and elevated plasma CRP levels. In female PCOS rats, Li K discovered that CRP insufficiency led to reduced leptin resistance and weight gain, enhanced insulin sensitivity and increased energy expenditure [36]. However, when TNF-α is persistently expressed at high levels in chronic inflammatory states, it triggers tissue damage and is capable of inducing metabolic disorders, particularly in metabolic conditions like obesity [37]. In the obese state, chronic low-grade inflammation is closely linked to high expression of TNF-α. Hypertrophic adipocytes and macrophages are the main producers of TNF-α in adipose tissue. Obese people have been discovered to have considerably higher levels of TNF-α, which is directly linked to endocrine dysfunction, lipid metabolic problems and insulin resistance [38]. TNF-α directly affects insulin sensitivity by blocking key players in insulin signaling (the insulin receptor substrate IRS-1) from becoming phosphorylated, which ultimately leads to the emergence of insulin resistance.
Furthermore, TNF-α's synergistic interaction with other inflammatory agents is a major contributor in metabolic problems associated with obesity. Through the production of multiple pro-inflammatory cytokines, which in turn produce an inflammatory feedback loop that affects metabolic balance or TNF-α exacerbates the chronic inflammatory state in obese patients. Chronic inflammation over an extended period of time not only causes insulin resistance but also encourages the emergence of further metabolic problems [39].
All things considered, the formation of metabolic disorders is significantly influenced with TNF-α, a primary mediator of chronic inflammation linked to obesity. Because it regulates the immunological response, promotes the production of inflammatory mediators and exacerbates metabolic imbalances, it may be a target for therapy of obesity and its associated effects.
Changes in TNF-α expression in obesity
An important factor in establishing insulin resistance and metabolic diseases, TNF-α is a primary mediator of obesity-associated chronic inflammation and is markedly raised in the adipose tissue of obese patients, particularly in visceral adipose. Studies have indicated that obesity is connected to the growth of adipocytes as well as the entry of macrophages into adipose tissue, which is the main source of TNF-α [40]. In addition, these adipocytes and macrophages release a lot of TNF-α, which aggravates the inflammatory response and interferes with the metabolism of adipose tissue.
TNF-α affects metabolism in a variety of ways by regulating a number of signaling pathways, particularly the modulation of insulin signaling. Insulin resistance is caused by TNF-α blocking the phosphorylation of insulin receptor substrate-1 (IRS-1), which prevents insulin from acting as it should [40, 41]. Visceral adipose tissue has significantly higher levels of TNF-α expression than subcutaneous adipose tissue, which explains why the patients with visceral obesity are more likely to develop metabolic syndrome. This mechanism is especially noticeable in obese patients, especially those who have visceral obesity. In addition to larger adipocytes, which the study found that obesity is linked to a considerable increase in macrophages in adipose tissue. These macrophages generated a vicious cycle that perpetuates inflammation and metabolic disorders by secreting TNF-α, which exacerbated the inflammatory response of adipocytes [41]. A chronic inflammatory state is maintained as a result of this vicious cycle, which eventually has an adverse effect on systemic metabolism.
In addition to being strongly linked to insulin resistance linked to obesity, that overexpression of TNF-α can also result in other metabolic disorders like diabetes and cardiovascular disease [42]. These studies further emphasize the critical role of TNF-α in obesity associated metabolic diseases and provide new targets for future therapeutic strategies.
Relationship between TNF-α and obesity-related metabolic disorders
TNF-α is strongly associated with obesity-related insulin resistance, a phenomenon first proposed by Hotamisligil et al. in 1993 [43]. They found that elevated levels of TNF-α in obese mice occurred in tandem with the emergence of insulin resistance and that further genetic blockade of the TNF-α signaling pathway could partially reverse the insulin resistance phenomenon. Since then, several studies have confirmed the important role of TNF-α in obesity-related metabolic abnormalities, especially insulin resistance. For instance, Xu H. demonstrated that a possible mechanism resulting in enhanced membrane-associated TNF-α in obese individuals' adipose tissue could be a decreased rate of mTNF-α processing in mature adipocytes along with increased TNF-α synthesis [44].
Insulin resistance is the result of TNF-α interfering with insulin signaling in a number of ways. One of the primary explanations is that TNF-α interferes with the PI3K/Akt activation process in the insulin signaling pathway by inhibiting the phosphorylation of insulin receptor substrate (IRS). Specifically, TNF-α blocks tyrosine phosphorylation of the insulin receptor by prompting serine phosphorylation of IRS-1 (especially at the Ser307 and Ser312 sites), which interferes with its normal binding to the insulin receptor. Insulin resistance results from this mechanism, which ultimately prevents the PI3K/Akt pathway from being activated and decreases the transport of the glucose transporter protein GluT4. Reduced glucose absorption, decreased insulin sensitivity, and eventually metabolic diseases are caused by dysregulation of the insulin signaling pathway [45, 46]. Furthermore, Plomgaard P demonstrated that TNF-α has a broad impact on a range of metabolic tissues by demonstrating that it not only impacts adipose tissue but also, via a comparable mechanism, leads to skeletal muscle insulin resistance [47].
In addition, TNF-alpha increases blood levels of free fatty acids by promoting lipolysis, which further exacerbates insulin resistance and metabolic abnormalities. This process exacerbates insulin resistance through several mechanisms. First, TNF-α promotes lipolysis by activating cAMP-dependent protein kinase (PKA), which inhibits lipid droplet protective proteins (e.g., Perilipin) in adipocytes. Elevated free fatty acids may intensify hepatic gluconeogenesis, impairing insulin signaling pathways and exacerbating insulin resistance [7, 48].
More recent research shown the vital role of the crucial function of TNF-α in controlling the metabolic syndrome associated with obesity.With activating the NF-κB signaling system, TNF-α stimulates the production of several pro-inflammatory proteins, exacerbating chronic low-grade inflammation. This inflammatory state not only affects insulin sensitivity, but also drives disorders of lipid metabolism and hepatic fat accumulation, leading to the development of nonalcoholic fatty liver disease (NAFLD). Experimental evidence through Vachliotis, I.D. has shown that TNF-α is a cytokine that plays a key role in the pathogenesis of NAFLD [49]. These metabolic abnormalities are particularly prominent in obese and PCOS patients, suggesting a key role for TNF-α in the regulation of energy metabolism, insulin sensitivity and lipid metabolism.
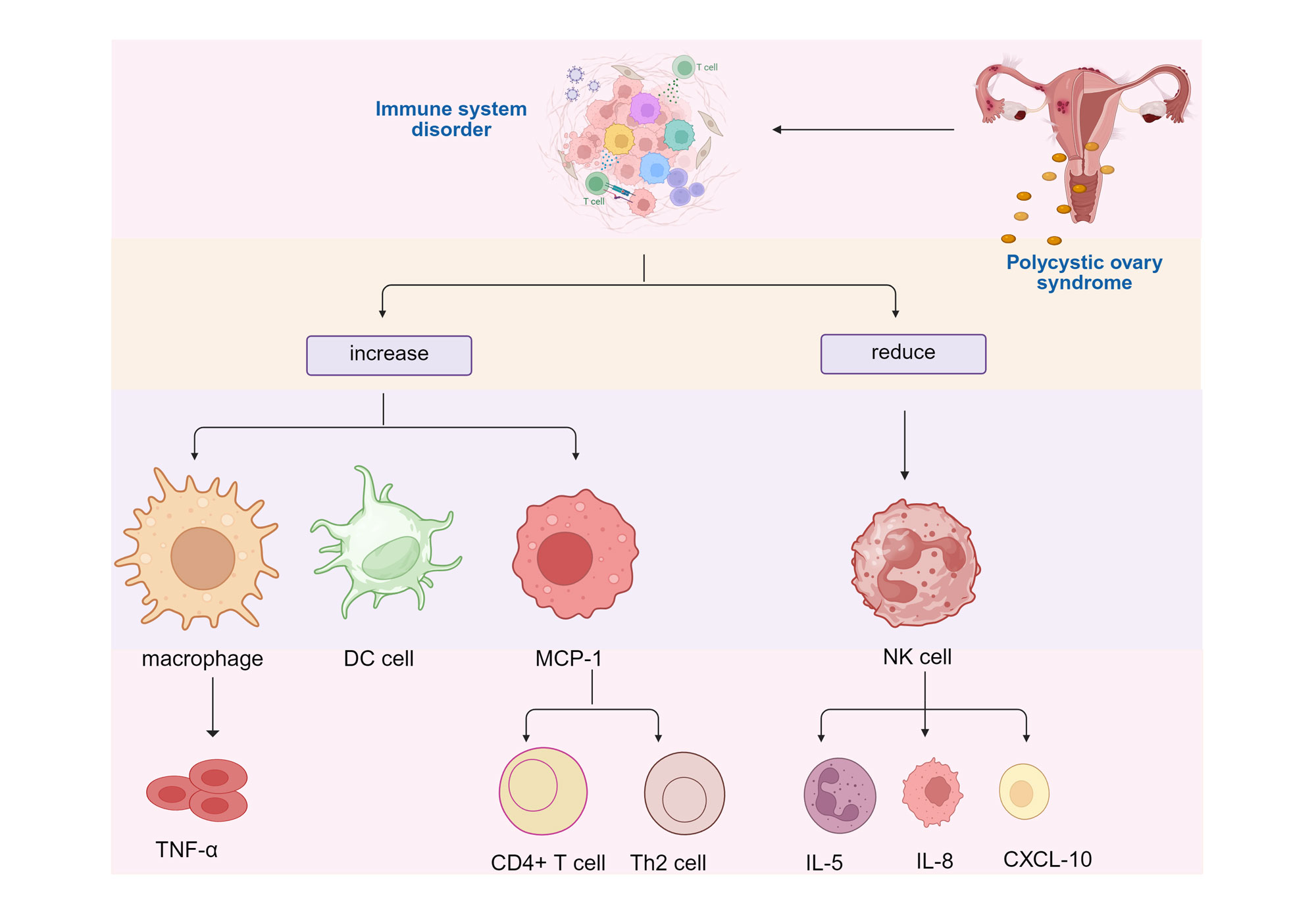 Figure 1. Impact of PCOS on Immune System Activation. PCOS induces immune system disorders, leading to an increase in pro-inflammatory cells (such as macrophages, DC cells) and inflammatory factors (such as TNF-α, MCP-1), while anti-inflammatory/immune monitoring cells (such as NK cells) and their related factors decrease, ultimately exacerbating the pathological process. DC cell: Dendritic Cell; MCP-1: Monocyte Chemoattractant Protein-1; NK cell: Natural Killer Cell; TNF-α: Tumor Necrosis Factor-alpha; IL-5: Interleukin-5; IL-8: Interleukin-8; CXCL-10: Chemokine Ligand 10; PCOS: Polycystic ovary syndrome.
Figure 1. Impact of PCOS on Immune System Activation. PCOS induces immune system disorders, leading to an increase in pro-inflammatory cells (such as macrophages, DC cells) and inflammatory factors (such as TNF-α, MCP-1), while anti-inflammatory/immune monitoring cells (such as NK cells) and their related factors decrease, ultimately exacerbating the pathological process. DC cell: Dendritic Cell; MCP-1: Monocyte Chemoattractant Protein-1; NK cell: Natural Killer Cell; TNF-α: Tumor Necrosis Factor-alpha; IL-5: Interleukin-5; IL-8: Interleukin-8; CXCL-10: Chemokine Ligand 10; PCOS: Polycystic ovary syndrome.
Clinical association study of leptin receptor and PCOS
Particularly in obese PCOS patients, the involvement of leptin in PCOS has drawn a lot of interest. Recent studies have shown that plasma leptin levels are substantially higher in PCOS patients and that these levels are strongly associated with leptin resistance and increased body fat. Nevertheless, even with increased leptin levels, PCOS patients still struggle greatly with energy metabolism and weight control, which may indicate leptin resistance.
Defects in leptin signaling are the primary characteristic of leptin resistance, a condition that is especially common in PCOS patients. Peng et al. discovered a substantial correlation between PCOS and highly increased blood leptin [52]. Obesity and high androgen levels work together to further exacerbate metabolic abnormalities in PCOS patients [53]. Manifestations of leptin resistance include diminished appetite regulation and decreased energy expenditure, which leads to difficulties in weight management.
Despite elevated leptin levels, its binding to the hypothalamic leptin receptor LEPR and subsequent signaling fails to function effectively. This abnormal function of LEPR may further exacerbate appetite control dysregulation, energy under-expenditure and metabolic imbalance by inhibiting the activation of the JAK-STAT signaling pathway [54]. In patients with PCOS, defective leptin receptor signaling not only exacerbates obesity, but is also strongly associated with their reproductive dysfunction, particularly due to abnormal leptin effects on the hypothalamic-pituitary-gonadal axis. Findings by Alexandra et. al. suggest that in a mouse model of PCOS-like phenotype, a subpopulation of AR/LepR cells mediates the effects of prenatal androgen excess on the female estrous cycle [55]. PCOS can affect immune function, cardiovascular health, and intestinal function, leading to immune abnormalities in the endometrium and follicular fluid (Figure 2).
In conclusion, clinical research has shown a substantial correlation between leptin levels in PCOS patients and insulin resistance, aberrant body fat distribution and other metabolic markers. Additionally, obese PCOS patients had increased insulin resistance and leptin levels, which supporting the pivotal role that leptin and its receptor play in metabolic problems associated with PCOS. Furthermore, reduced fertility in PCOS patients was strongly linked to altered leptin receptor activity, which indicating that irregularities in the leptin signaling pathway may cause ovarian function and reproductive hormone secretion to be affected, ultimately leading to ovulatory dysfunction in PCOS patients.
TNF-α and the pathogenesis of PCOS
Strong pro-inflammatory cytokine TNF-α has received a lot of interest due to its role in the pathophysiology of PCOS. Studies have shown that people with PCOS, especially those who are obese, have much higher levels of TNF-α.through a number of routes. TNF-α not only promotes a chronic low-grade inflammatory state but also plays a role in the metabolic issues and reproductive dysfunctions associated with PCOS.
First of all, TNF-α contributes to the development of insulin resistance by directly interfering with insulin signaling. Through preventing the phosphorylation of the insulin receptor substrate (IRS-1), which prevents the activation of the insulin signaling pathway, TNF-α aggravates obesity and metabolic diseases. This lowers glucose uptake and utilization, which eventually results in decreased insulin sensitivity [56]. TNF-α expression is linked to increasing body fat in obese people, and the onset of diabetes and other metabolic disorders is frequently accompanied by elevated TNF-α levels [7]. As metabolic imbalance exacerbated by elevated TNF-α levels, one of the main characteristics of PCOS is insulin resistance.
Furthermore, TNF-α also significantly affects PCOS patients' ovarian function. Research has indicated that TNF-α causes hyperandrogenemia and ovulatory dysfunction in the ovary by interfering with androgen production and ovulatory processes through its pro-inflammatory actions. Through triggering the ovarian cells' NF-κB signaling pathway and controlling the expression of numerous genes linked to reproductive function, TNF-α impedes the process of follicular development and maturation. This pro-inflammatory mechanism exacerbates the reproductive hormone imbalance in PCOS patients, further contributing to the symptoms of anovulation and infertility. Abraham et al. demonstrated that the interactions indicates that inflammation is one of the most powerful risk factors for PCOS, which as evidenced by the correlation between inflammatory cytokines in the PCOS ovary [57].
In addition, according to the meta-analysis by Gao et al., TNF-alpha levels were also considerably higher in PCOS patients than in healthy controls. This suggests that TNF-alpha could be a possible biomarker for PCOS, reflecting its metabolic and reproductive abnormalities (PLOS) [58]. These results demonstrate the possibility for therapeutic targeting of TNF-α and support the critical role of TNF-α in the pathophysiology of PCOS, particularly in obese persons, where the chronic inflammation it fosters is intimately linked to metabolic abnormalities.
Effect of obesity on leptin and TNF-α levels in patients with PCOS
Leptin and TNF-α are important mediators in the complex link between obesity and PCOS, which is exacerbated by a number of processes. In obese individuals, adipose tissue serves as an energy storage organ and an endocrine organ that secretes TNF-α and leptin among other metabolic regulators. In PCOS linked to obesity, a chronic inflammatory state in adipose tissue is more pronounced, leading to an overproduction of leptin and TNF-α.
Scientific study has indicated that people with PCOS who are obese and have high leptin levels generally have considerably reduced leptin resistance, which impairs their ability to regulate energy metabolism and suppress appetite. It's possible that inflammation, insulin resistance and hyperandrogenism are closely linked to the process of leptin resistance [59]. Because of this resistance, appetite increases and energy expenditure decreases which exacerbates obesity and the related metabolic issues [60, 61].
In obese patients, TNF-α is a primary pro-inflammatory cytokine and is crucial to the pathophysiology of PCOS. Chronic low-grade inflammation linked to obesity facilitates elevated TNF-α release, which in turn drives insulin resistance and elevated testosterone levels [62]. Studies have demonstrated that TNF-α increases metabolic irregularities and decreases insulin bioactivity by interfering with the insulin receptor signaling system. The chronic inflammatory state in adipose tissue is more pronounced in obese PCOS patients, which increases TNF-α and leptin secretion and causes insulin resistance, increased blood glucose and associated metabolic syndrome. In addition to impairing ovarian function, the chronic inflammatory condition stimulates the production of endogenous hormones, which results in irregular menstruation and decreased fertility [59]. PCOS is more difficult to treat when there is obesity because it exacerbates the metabolic irregularities and reproductive dysfunction through two main pathways: leptin and TNF-α.
Interaction of leptin with TNF-α
Leptin and TNF-α interact significantly in the pathophysiology of PCOS. According to research, these two factors have a mutually reinforcing association in inflammatory states associated with obesity. As a result, people with PCOS experience a worsening of their metabolic dysregulation and infertility [63].
Leptin and TNF-α promote the release of inflammatory chemicals and maintain the chronic inflammatory state associated with obesity by activating pro-inflammatory signaling pathways including NF-κB. The persistent inflammation has an impact on insulin signaling and ovarian function, which leads to an imbalance in the release of reproductive hormones. Studies have indicated a significant association between elevated levels of leptin and TNF-α and insulin resistance, with the latter phenomenon being particularly noticeable in obese PCOS patients [64, 65].
Furthermore, In PCOS patients the combined effects of leptin and TNF-α can significantly affect reproductive function. The ovary's leptin receptor and TNF-α regulate hormone release and follicular development through a range of signaling pathways. An imbalance in ovarian reproductive hormones, brought by dysregulated leptin signaling or high TNF-α levels, leads to ovulatory dysfunction and hyperandrogenemia. This dual dysregulation of metabolism and reproduction forms an important part of the pathologic profile of patients with PCOS [63].
In addition, obese patients have considerably higher levels of inflammatory markers including TNF-α and C-reactive protein (CRP) but PCOS is also closely linked to chronic low-grade inflammation. Even in women of normal weight, levels of inflammatory markers like TNF-α, IL-6, and IL-18 are considerably higher in PCOS patients than in healthy controls, indicating that the inflammatory state of PCOS may exist independently of body weight and is not just associated with obesity [64], [66].
Therefore, therapeutic interventions targeting leptin and TNF-α may provide new therapeutic ideas to improve metabolic and reproductive problems in PCOS patients. The symptoms of PCOS can be successfully reduced and patients' quality of life can be enhanced, with lowering the inflammatory state and enhancing hormonal balance. The main consequences caused by TNF-α are summarized in Table 1.
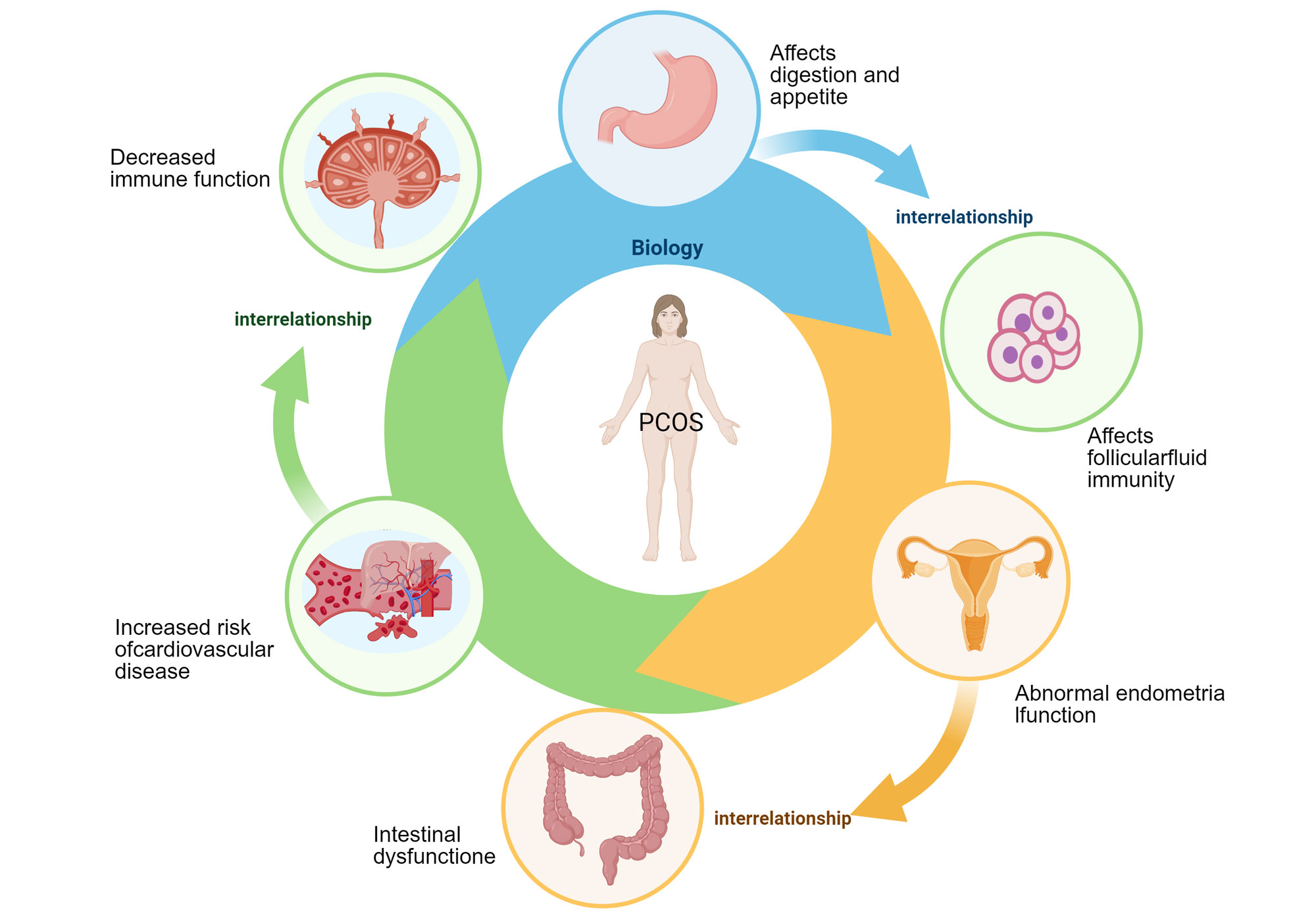 Figure 2. Interrelated health impacts of PCOS on various bodily systems. Different organ systems such as the stomach, ovaries, uterus, intestines, liver, and adrenal glands interact with each other to form a circulatory network and are impacted by PCOS. Specifically, abnormal gastric function affects digestive function and appetite, affects follicular fluid immunity, abnormal endometrial function in the uterus, and "functional impairment" in the intestines are associated with an increased risk of cardiovascular disease. Overall, complex impact of PCOS on multiple systems throughout the body and the interactions between these systems is evident.
Figure 2. Interrelated health impacts of PCOS on various bodily systems. Different organ systems such as the stomach, ovaries, uterus, intestines, liver, and adrenal glands interact with each other to form a circulatory network and are impacted by PCOS. Specifically, abnormal gastric function affects digestive function and appetite, affects follicular fluid immunity, abnormal endometrial function in the uterus, and "functional impairment" in the intestines are associated with an increased risk of cardiovascular disease. Overall, complex impact of PCOS on multiple systems throughout the body and the interactions between these systems is evident.
|
Table 1. Main effects of TNF-α production. |
|||
|
TNF-α main effects |
Pathway |
Results |
Reference |
|
Promote chronic inflammation |
NF-κB pathway |
Exacerbates chronic low-grade inflammation leading to insulin resistance and chronic metabolic disorders |
[40, 41] |
|
Interferes with insulin signaling |
PI3K/Akt disruption |
Decreases glucose uptake and insulin sensitivity by inhibiting insulin receptor substrate (IRS-1) phosphorylation |
[41] |
|
Induction of lipolysis |
PKA activation |
Promotes lipolysis and increases serum free fatty acids, further aggravating insulin resistance |
[48] |
|
Impact on ovarian function |
Regulation of inflammatory gene expression |
Activates pro-inflammatory factor NF-κB interferes with follicular development, leading to hyperandrogenism and impaired ovulation |
[7], [56-58] |
|
Synergizes with other pro-inflammatory factors |
Multiple pro-inflammatory signaling pathways interact |
By synergizing with pro-inflammatory factors such as IL-6 and IL-1β, an inflammatory feedback loop is formed, exacerbating metabolic abnormalities |
[13], [64], [66] |
|
Effects on hepatic lipid metabolism |
NF-κB and other inflammatory pathways |
Promotes disorders of hepatic lipid metabolism that may lead to nonalcoholic fatty liver disease (NAFLD) |
[13], [41] |
Second, as a fundamental mediator of chronic low-grade inflammation linked to obesity, TNF-α is also essential for the development of PCOS. Patients with PCOS, particularly those who are obese, have significantly higher levels of TNF-α. This inflammatory condition impacts the NF-κB signaling pathway in the ovaries in addition to directly interfering with the insulin signaling pathway, which leads to insulin resistance by activating the hormone secretion and follicular development in the ovary, leading to hyperandrogenemia and ovulatory dysfunction [7], [57], [70]. These findings suggest that TNF-α is not limited to metabolic regulation in the pathomechanism of PCOS, but also profoundly affects reproductive function in PCOS patients through its pro-inflammatory effects. In addition, leptin and TNF-α show a complex interaction in obesity-related PCOS. The two form a mutually reinforcing positive feedback loop in the obese state, and together they maintain a chronic low-grade inflammatory state. Chronic inflammation not only adds to the disruption of leptin signaling, but also further exacerbates insulin resistance and metabolic disorders [1], [71], [72]. In summary, the interaction between leptin and TNF-α in the obese state is one of the key mechanisms of metabolic and reproductive abnormalities in PCOS; therefore, interventions targeting the interaction between the two may provide new directions for the treatment of PCOS.
Although the pro-inflammatory role of TNF-α in PCOS has been widely investigated, its mechanism of action and targets in different tissues (e.g., adipose tissue and ovary) still need to be further explored. In the future, we should focus on the specific regulation of TNF-α in different tissues of PCOS patients and its interactions with other inflammatory factors, and we should also pay more attention to the interaction mechanism between leptin and TNF-α and its dual effects on metabolic and reproductive functions. Leptin resistance is particularly pronounced in PCOS patients, but its specific molecular mechanisms have not been fully elucidated. Future studies should focus on the regulatory mechanisms of the leptin receptor signaling pathway, especially the role of the JAK-STAT pathway and its negative feedback regulators (e.g., SOCS3). In addition, exploring how to restore leptin signaling function and thus improve the leptin resistance status is one of the key directions for future research. In addition, there is also the consideration of how to conduct early detection of PCOS patients, and according to Fang Zenghui's study, the results showed that anti-Müllerian hormone (AMH) as well as lipid level testing have clinical significance in the early diagnosis of PCOS [73].
Leptin and TNF-α have complex interactions in obesity-associated PCOS patients, and joint modulation of leptin and TNF-α signaling pathways could be considered in the future to reduce inflammatory status and improve metabolic abnormalities and reproductive function. For example, by targeting and inhibiting the pro-inflammatory signaling of TNF-α or enhancing leptin signaling, it may help to alleviate the symptoms and improve the quality of life of PCOS patients. Given the important role of obesity in the pathomechanism of PCOS, personalized therapeutic strategies should be developed for PCOS patients with different body sizes and pathological features. For example, for obese PCOS patients, the focus should be on weight reduction and alleviation of chronic inflammation, whereas for non-obese PCOS patients, more attention to the modulation of their leptin and TNF-α levels may lead to better therapeutic outcomes.
In conclusion, leptin receptor and TNF-α jointly maintain a chronic inflammatory state that contributes to metabolic and reproductive abnormalities in PCOS. These processes are crucial to the pathomechanism of obesity-associated PCOS. Thus, focusing on the leptin and TNF-α signaling modulation may offer fresh approaches to enhance PCOS treatment. Future research should focus on understanding the molecular mechanisms underlying leptin resistance and the function of TNF-α in various tissues in order to offer a stronger scientific foundation for treating PCOS on an individual basis.
No applicable.
Ethics approval
No applicable.
Data availability
This narrative review is based on previously published studies and publicly available data. No new datasets were generated or analyzed for the current review.
Funding
None.
Authors’ contribution
OMA contributed to the design, writing, collected data and drew figures for the manuscript. AAAA revised the manuscript.
Competing interests
The authors declare no competing interests.
- Zhao H, Zhang J, Cheng X, Nie X, He B: Insulin resistance in polycystic ovary syndrome across various tissues: an updated review of pathogenesis, evaluation, and treatment. J Ovarian Res 2023, 16(1): 9.
- Zhang H, Wang W, Zhao J, Jiao P, Zeng L, Zhang H, Zhao Y, Shi L, Hu H, Luo L et al: Relationship between body composition, insulin resistance, and hormonal profiles in women with polycystic ovary syndrome. Front Endocrinol (Lausanne) 2022, 13: 1085656.
- Mansour A, Noori M, Hakemi MS, Haghgooyan Z, Mohajeri-Tehrani MR, Mirahmad M, Sajjadi-Jazi SM: Hyperandrogenism and anthropometric parameters in women with polycystic ovary syndrome. BMC Endocr Disord 2024, 24(1): 201.
- Fahs D, Salloum D, Nasrallah M, Ghazeeri G: Polycystic Ovary Syndrome: Pathophysiology and Controversies in Diagnosis. Diagnostics 2023, 13(9): 1559.
- Jurczewska J, Ostrowska J, Chełchowska M, Panczyk M, Rudnicka E, Kucharski M, Smolarczyk R, Szostak-Węgierek D: Abdominal Obesity in Women with Polycystic Ovary Syndrome and Its Relationship with Diet, Physical Activity and Insulin Resistance: A Pilot Study. Nutrients 2023, 15(16): 3652.
- Carmina E, Bucchieri S, Esposito A, Del Puente A, Mansueto P, Orio F, Di Fede G, Rini G: Abdominal Fat Quantity and Distribution in Women with Polycystic Ovary Syndrome and Extent of Its Relation to Insulin Resistance. J Clin Endocrinol Metab 2007, 92(7): 2500-2505.
- Dey R, Bhattacharya K, Basak AK, Paul N, Bandyopadhyay R, Chaudhuri GR, Purkait MP, Bhattacharjee A, Bose C, Shukla N: Inflammatory perspectives of polycystic ovary syndrome: role of specific mediators and markers. Mid East Fert Soc J 2023, 28(1): 33.
- Liu Z, Xiao T, Liu H: Leptin signaling and its central role in energy homeostasis. Front Neurosci 2023, 17: 1238528.
- Izquierdo AG, Crujeiras AB, Casanueva FF, Carreira MC: Leptin, Obesity, and Leptin Resistance: Where Are We 25 Years Later? Nutrients 2019, 11(11): 2704.
- Banerjee S, Cooney LG, Stanic AK: Immune Dysfunction in Polycystic Ovary Syndrome. ImmunoHorizons 2023, 7(5):323-332.
- Hotamisligil GS, Budavari A, Murray D, Spiegelman BM: Reduced tyrosine kinase activity of the insulin receptor in obesity-diabetes. Central role of tumor necrosis factor-alpha. J Clin Invest 1994, 94(4): 1543-1549.
- González F, Rote NS, Minium J, Kirwan JP: Increased Activation of Nuclear Factor κB Triggers Inflammation and Insulin Resistance in Polycystic Ovary Syndrome. J Clin Endocrinol Metab 2006, 91(4): 1508-1512.
- Vasyukova E, Zaikova E, Kalinina O, Gorelova I, Pyanova I, Bogatyreva E, Vasilieva E, Grineva E, Popova P: Inflammatory and Anti-Inflammatory Parameters in PCOS Patients Depending on Body Mass Index: A Case-Control Study. Biomedicines 2023, 11(10):2791.
- Misch M, Puthanveetil P: The Head-to-Toe Hormone: Leptin as an Extensive Modulator of Physiologic Systems. International Journal of Molecular Sciences 2022, 23(10):5439.
- Morselli LL, Claflin KE, Cui H, Grobe JL: Control of Energy Expenditure by AgRP Neurons of the Arcuate Nucleus: Neurocircuitry, Signaling Pathways, and Angiotensin. Curr Hypert Rep 2018, 20(3): 25.
- Caron A, Dungan Lemko HM, Castorena CM, Fujikawa T, Lee S, Lord CC, Ahmed N, Lee CE, Holland WL, Liu C, Elmquist JK: POMC neurons expressing leptin receptors coordinate metabolic responses to fasting via suppression of leptin levels. eLife 2018, 7: e33710.
- Bakhtyukov AA, Lebedev IA, Kuznetsova VS, Derkach KV, Shpakov AO: Leptin Fragment 116–122 Modulates Testicular Steroidogenesis in Male Rats. J Evolut Biochem Physiol 2023, 59(3):904-913.
- Ballauff A, Ziegler A, Emons G, Sturm G, Blum WF, Remschmidt H, Hebebrand J: Serum leptin and gonadotropin levels in patients with anorexia nervosa during weight gain. Molecular Psychiatry 1999, 4(1): 71-75.
- Jamal MA, Cheng Y, Jiao D, Cheng W, Zou D, Wang X, Wei T, Guo J, Xu K, Zhao H et al: Unraveling the impact of hyperleptinemia on female reproduction: insights from transgenic pig model. Biol Res 2024, 57(1): 60.
- Dam J, Jockers R, Guerre-Millo M, Clément K: Leptin Receptors and Mechanism of Action. In: Leptin: Regulation and Clinical Applications. Edited by Dagogo-Jack MDS. Cham: Springer International Publishing; 2015: 15-24.
- Jin L, Burguera BG, Couce ME, Scheithauer BW, Lamsan J, Eberhardt NL, Kulig E, Lloyd RV: Leptin and Leptin Receptor Expression in Normal and Neoplastic Human Pituitary: Evidence of a Regulatory Role for Leptin on Pituitary Cell Proliferation1. J Clin Endocrinol Metab 1999, 84(8): 2903-2911.
- Knobelspies H, Zeidler J, Hekerman P, Bamberg-Lemper S, Becker W: Mechanism of attenuation of leptin signaling under chronic ligand stimulation. BMC Biochem 2010, 11(1): 2.
- Wang P, Yang F-J, Du H, Guan Y-F, Xu T-Y, Xu X-W, Su D-F, Miao C-Y: Involvement of Leptin Receptor Long Isoform (LepRb)-STAT3 Signaling Pathway in Brain Fat Mass- and Obesity-Associated (FTO) Downregulation during Energy Restriction. Mol Med 2011, 17(5): 523-532.
- Wauman J, Tavernier J: Leptin receptor signaling: pathways to leptin resistance. Front Biosci (Landmark Ed) 2011, 16(7): 2771-2793.
- Corbould A: Insulin resistance in skeletal muscle and adipose tissue in polycystic ovary syndrome: are the molecular mechanisms distinct from type 2 diabetes? Panminerva Med 2008, 50(4): 279-294.
- Solinas G, Becattini B: PI3K and AKT at the Interface of Signaling and Metabolism. Curr Top Microbiol Immunol 2022, 436: 311-336.
- Rhea EM, Salameh TS, Logsdon AF, Hanson AJ, Erickson MA, Banks WA: Blood-Brain Barriers in Obesity. AAPS J 2017, 19(4):921-930.
- Shi Y, Kim H, Hamann CA, Rhea EM, Brunger JM, Lippmann ES: Nuclear receptor ligand screening in an iPSC-derived in vitro blood–brain barrier model identifies new contributors to leptin transport. Fluids and Barriers of the CNS 2022, 19(1): 77.
- Casado ME, Collado-Pérez R, Frago LM, Barrios V: Recent Advances in the Knowledge of the Mechanisms of Leptin Physiology and Actions in Neurological and Metabolic Pathologies. International J Mol Sci 2023, 24(2): 1422.
- Casado ME, Collado-Pérez R, Frago LM, Barrios V: Recent Advances in the Knowledge of the Mechanisms of Leptin Physiology and Actions in Neurological and Metabolic Pathologies. Int J Mol Sci 2023, 24(2): 1422.
- Lee MJ, Wang Y, Ricci MR, Sullivan S, Russell CD, Fried SK: Acute and chronic regulation of leptin synthesis, storage, and secretion by insulin and dexamethasone in human adipose tissue. Am J Physiol Endocrinol Metab 2007, 292(3): E858-864.
- Genchi VA, D’Oria R, Palma G, Caccioppoli C, Cignarelli A, Natalicchio A, Laviola L, Giorgino F, Perrini S: Impaired Leptin Signalling in Obesity: Is Leptin a New Thermolipokine? Int J Mol Sci 2021, 22(12): 6445.
- Genchi VA, D'Oria R, Palma G, Caccioppoli C, Cignarelli A, Natalicchio A, Laviola L, Giorgino F, Perrini S: Impaired Leptin Signalling in Obesity: Is Leptin a New Thermolipokine? Int J Mol Sci 2021, 22(12): 6445.
- Reed AS, Unger EK, Olofsson LE, Piper ML, Myers MG, Jr., Xu AW: Functional role of suppressor of cytokine signaling 3 upregulation in hypothalamic leptin resistance and long-term energy homeostasis. Diabetes 2010, 59(4): 894-906.
- Obradovic M, Sudar-Milovanovic E, Soskic S, Essack M, Arya S, Stewart AJ, Gojobori T, Isenovic ER: Leptin and Obesity: Role and Clinical Implication. Front Endocrinol (Lausanne) 2021, 12:585887.
- Li K, Hu L, Li X, Yuan Z, He J, Liu D, Yang G, Yuan L: Effect of C-reactive protein deficiency on insulin resistance reversal in rats with polycystic ovary syndrome through augmented leptin action. Diabetol Metab Syndr 2023, 15(1): 180.
- Felipe H, Alexander HB, Miguel Luiz Batista J: Adipose Tissue Inflammation and Metabolic Disorders. In: Adipose Tissue. edn. Edited by Leszek S. Rijeka: IntechOpen; 2019: Ch. 5.
- Zatterale F, Longo M, Naderi J, Raciti GA, Desiderio A, Miele C, Beguinot F: Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front Physiol 2019, 10: 1607.
- Borst SE: The role of TNF-α in insulin resistance. Endocrine 2004, 23(2): 177-182.
- Cai Z, Huang Y, He B: New Insights into Adipose Tissue Macrophages in Obesity and Insulin Resistance. Cells 2022, 11(9): 1424.
- Li X, Ren Y, Chang K, Wu W, Griffiths HR, Lu S, Gao D: Adipose tissue macrophages as potential targets for obesity and metabolic diseases. Front Immunol 2023, 14: 1153915.
- Al-Mansoori L, Al-Jaber H, Prince MS, Elrayess MA: Role of Inflammatory Cytokines, Growth Factors and Adipokines in Adipogenesis and Insulin Resistance. Inflammation 2022, 45(1): 31-44.
- Hotamisligil GS, Shargill NS, Spiegelman BM: Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993, 259(5091): 87-91.
- Xu H, Uysal KT, Becherer JD, Arner P, Hotamisligil GS: Altered tumor necrosis factor-alpha (TNF-alpha) processing in adipocytes and increased expression of transmembrane TNF-alpha in obesity. Diabetes 2002, 51(6): 1876-1883.
- Ramasubbu K, Devi Rajeswari V: Impairment of insulin signaling pathway PI3K/Akt/mTOR and insulin resistance induced AGEs on diabetes mellitus and neurodegenerative diseases: a perspective review. Mol Cell Biochem 2023, 478(6): 1307-1324.
- Rui L, Aguirre V, Kim JK, Shulman GI, Lee A, Corbould A, Dunaif A, White MF: Insulin/IGF-1 and TNF-alpha stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J Clin Invest 2001, 107(2): 181-189.
- Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK: Tumor necrosis factor-alpha induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes 2005, 54(10): 2939-2945.
- Boden G: Free fatty acids, a major link between obesity, insulin resistance, inflammation, and atherosclerotic vascular disease. In: Cardiovascular Endocrinology: Shared Pathways and Clinical Crossroads. edn.: Springer; 2009: 61-70.
- Vachliotis ID, Polyzos SA: The Role of Tumor Necrosis Factor-Alpha in the Pathogenesis and Treatment of Nonalcoholic Fatty Liver Disease. Curr Obes Rep 2023, 12(3): 191-206.
- Vachliotis ID, Polyzos SA: The Role of Tumor Necrosis Factor-Alpha in the Pathogenesis and Treatment of Nonalcoholic Fatty Liver Disease. Curr Obes Rep 2023, 12(3): 191-206.
- Zhu T, Cui J, Goodarzi MO: Polycystic Ovary Syndrome and Risk of Type 2 Diabetes, Coronary Heart Disease, and Stroke. Diabetes 2021, 70(2): 627-637.
- Nayeem J, Islam MMT, Deeba F, Selim S, Ali L, Kabir Y: Insulin resistance and insulin secretory defect among Bangalee PCOS women: a case-control study. BMC Endocr Disord 2024, 24(1): 207.
- Polak AM, Krentowska A, Łebkowska A, Buczyńska A, Adamski M, Adamska-Patruno E, Fiedorczuk J, Krętowski AJ, Kowalska I, Adamska A: The Association of Serum Levels of Leptin and Ghrelin with the Dietary Fat Content in Non-Obese Women with Polycystic Ovary Syndrome. Nutrients 2020, 12(9): 2753.
- Liu H, Du T, Li C, Yang G: STAT3 phosphorylation in central leptin resistance. Nutr Metab 2021, 18(1): 39.
- Cara A, Burger LL, Myers MG, Gendt KD, Moenter S, Elias CF: SUN-012 Role of Leptin-Receptor Expressing Cells in the Pathogenesis of Polycystic Ovary Syndrome (PCOS). Journal of the Endocrine Society 2020, 4(Supplement_1).
- Popko K, Gorska E, Stelmaszczyk-Emmel A, Plywaczewski R, Stoklosa A, Gorecka D, Pyrzak B, Demkow U: Proinflammatory cytokines IL-6 and TNF-α and the development of inflammation in obese subjects. Eur J Med Res 2010, 15(2): 120.
- Abraham Gnanadass S, Divakar Prabhu Y, Valsala Gopalakrishnan A: Association of metabolic and inflammatory markers with polycystic ovarian syndrome (PCOS): an update. Arch Gynecol Obstet 2021, 303(3): 631-643.
- Gao L, Gu Y, Yin X: High Serum Tumor Necrosis Factor-Alpha Levels in Women with Polycystic Ovary Syndrome: A Meta-Analysis. PLoS One 2016, 11(10): e0164021.
- Aubuchon M, Bickhaus JA, González F: Obesity, Metabolic Dysfunction, and Inflammation in Polycystic Ovary Syndrome. In: Polycystic Ovary Syndrome: Current and Emerging Concepts. edn. Edited by Pal L. New York, NY: Springer New York; 2014: 117-144.
- Lonardo MS, Cacciapuoti N, Guida B, Di Lorenzo M, Chiurazzi M, Damiano S, Menale C: Hypothalamic-Ovarian axis and Adiposity Relationship in Polycystic Ovary Syndrome: Physiopathology and Therapeutic Options for the Management of Metabolic and Inflammatory Aspects. Curr Obes Rep 2024, 13(1): 51-70.
- Engin A: The mechanism of leptin resistance in obesity and therapeutic perspective. Adv Exp Med Biol 2024, 1460: 463-487.
- Regidor PA, Mueller A, Sailer M, Gonzalez Santos F, Rizo JM, Egea FM: Chronic Inflammation in PCOS: The Potential Benefits of Specialized Pro-Resolving Lipid Mediators (SPMs) in the Improvement of the Resolutive Response. Int J Mol Sci 2020, 22(1): 384.
- Sikiru AB, Adeniran MA, Akinola K, Behera H, Kalaignazhal G, Egena SSA: Unraveling the complexity of the molecular pathways associated with polycystic ovary syndrome (PCOS) and identifying molecular targets for therapeutic development: a review of literature. Mid East Fert Soci J 2023, 28(1): 16.
- Aboeldalyl S, James C, Seyam E, Ibrahim EM, Shawki HE, Amer S: The Role of Chronic Inflammation in Polycystic Ovarian Syndrome-A Systematic Review and Meta-Analysis. Int J Mol Sci 2021, 22(5): 2734.
- Szukiewicz D, Trojanowski S, Kociszewska A, Szewczyk G: Modulation of the Inflammatory Response in Polycystic Ovary Syndrome (PCOS)-Searching for Epigenetic Factors. Int J Mol Sci 2022, 23(23): 14663.
- Aboeldalyl S, James C, Seyam E, Ibrahim EM, Shawki HE-D, Amer S: The Role of Chronic Inflammation in Polycystic Ovarian Syndrome—A Systematic Review and Meta-Analysis. Int J Mol Sci 2021, 22(5): 2734.
- Liao B, Qiao J, Pang Y: Central Regulation of PCOS: Abnormal Neuronal-Reproductive-Metabolic Circuits in PCOS Pathophysiology. Front Endocrinol (Lausanne) 2021, 12: 667422.
- Chou SH, Mantzoros C: 20 YEARS OF LEPTIN: Role of leptin in human reproductive disorders. J Endocrinol 2014, 223(1): T49-T62.
- Chan JL, Mantzoros CS: Leptin and the Hypothalamic-Pituitary Regulation of the Gonadotropin-Gonadal Axis. Pituitary 2001, 4(1): 87-92.
- Kar TK, Sil S, Ghosh A, Barman A, Chattopadhyay S: Mitigation of letrozole induced polycystic ovarian syndrome associated inflammatory response and endocrinal dysfunction by Vitex negundo seeds. J Ovarian Res 2024, 17(1): 76.
- Rehman K, Akash MS: Mechanisms of inflammatory responses and development of insulin resistance: how are they interlinked? J Biomed Sci 2016, 23(1): 87.
- Goldsammler M, Merhi Z, Buyuk E: Role of hormonal and inflammatory alterations in obesity-related reproductive dysfunction at the level of the hypothalamic-pituitary-ovarian axis. Reprod Biol Endocrinol 2018, 16(1): 45.
- Rudnicka E, Suchta K, Grymowicz M, Calik-Ksepka A, Smolarczyk K, Duszewska AM, Smolarczyk R, Meczekalski B: Chronic Low Grade Inflammation in Pathogenesis of PCOS. Int J Mol Sci 2021, 22(7): 3789.
Asia-Pacific Journal of Surgical & Experimental Pathology
ISSN 2977-5817 (Online)
 Copyright © Asia Pac J Surg Exp & Pathol. This
work is licensed under a Creative Commons AttributionNonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0)
License.
Copyright © Asia Pac J Surg Exp & Pathol. This
work is licensed under a Creative Commons AttributionNonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0)
License.

 Submit Manuscript
Submit Manuscript